Please review our publications to fully explore our research.
Motivation
Background
| Cartilage is a highly specialized musculoskeletal tissue that serves a number of functions in the human body. In general, cartilage acts to distribute loads across joints, to facilitate the frictionless movement of joints, and to absorb shock. Unlike most tissues in the human body, cartilage is, for the most part, avascular, relying on diffusion to nourish the few cells found in cartilage, called chondrocytes. Chondrocytes themselves are unique cells, adapted to living under conditions of low oxygen tension and high mechanical loads. Surrounding the cells is the extracellular matrix (ECM) that gives cartilage its unique properties. Most cartilaginous tissues contain fibrillar proteins called collagens, and macromolecules called proteoglycans. One of the most intriguing and problematic features of cartilage is that it has very little capacity for self-repair. When cartilage does manage to heal, the resulting repair tissue is biomechanically inferior, and rapidly degrades under regular use. As a result, cartilage has been the target of intensive research in an effort to develop tissue engineering approaches to regenerate it. Articular cartilage, in particular, has been studied by a number of institutions and corporations worldwide. More recently, other tissues, like the knee meniscus and the temporomandibular joint (TMJ) disc, have also been targeted for potential tissue engineered replacement. However, no one has satisfactorily replicated the functional biomechanical properties of these cartilaginous tissues. | 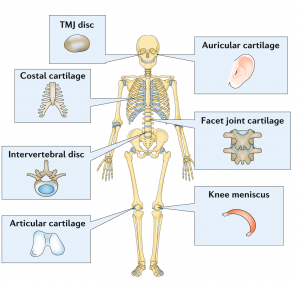 Adapted from Bielajew, Hu, Athanasiou, 2020. Adapted from Bielajew, Hu, Athanasiou, 2020. |
Our Research
| The main objectives of our research are (1) to develop a fundamental understanding of the cellular, biochemical, and biomechanical characteristics of articular cartilage, the knee meniscus, and the TMJ disc, and (2) to use this information to develop rational and novel approaches for tissue engineering. Recently, we have pioneered the use of self-assembly to create functional constructs without the use of scaffolds. Self-assembly has been improved by applying exogenous stimuli such as hydrostatic pressure and growth factors to enhance construct functional properties. Additionally, alternate cell sources including dermis-derived cells and human embryonic stem cells have been investigated to make this technology more translatable. | 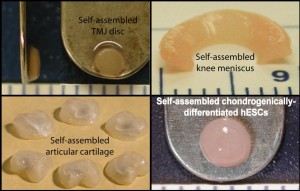 |

