
Current Projects:
1 – “Probing the Surfaces of Atmospheric Organic Particles and the Implications for Climate Change, Air Quality, Visibility and Bioavailabilty”
Atmospheric organic particles are rarely uniform in composition. That is, the molecules that initiated the formation of the particle may have different functional groups and volatilities from those on its surface. This composition difference can arise for a variety of reasons: their phase, which affects mixing and condensed-phase chemistry; their growth from gas-phase partitioning into the particles; their uptake and loss of water as they move through the atmosphere; and the atmospheric chemistry and photochemistry that continues to occur at their surfaces as they age.
The goal of this research is to examine the surface layers of well-defined model particle systems in the laboratory using a variety of analytical techniques and determine how this surface differs from the particle core. Comparison of the surface composition with the bulk or average composition will provide insights on how organic particles reduce visibility, how efficiently they lead to cloud formation, and how they interact with biological systems.

“Probing Surfaces of Atmospherically Relevant Organic Particles by Easy Ambient Sonic-Spray Ionization Mass Spectrometry (EASI-MS)”, L. Wingen et al., Chemical Science (2019) – link

DART-MS source
“New insights into atmospherically relevant reaction systems using direct analysis in real time-mass spectrometry (DART-MS)”, Y. Zhao et al., AMTD (2016) link

EESI-MS source
“Analysis of secondary organic aerosols in air using extractive electrospray ionization mass spectrometry”, L. Doezema et al., RSC Adv. (2012) link
“New mechanism of extractive electrospray ionization mass spectrometry for heterogeneous solid particles”, S. Kumbhani et al., Anal. Chem. (2018) link
2 – “Developing the Molecular Underpinning for Mechanisms of Growth of Secondary Organic Aerosol (SOA) Particles in Air”
This project seeks to investigate the mechanism behind particle growth via reactions between compounds commonly found in the atmosphere. One reaction system is that between amines and dicarboxylic acids (diacids). The reaction is examined using a Knudsen cell coupled to a quadrupole mass spectrometer. The gas phase amine reacts with the solid diacid and from there, an uptake coefficient can be estimated. Experiments show an interesting odd-even carbon effect towards their reaction with amines, with odd carbon diacids exhibiting larger uptake coefficients. The reason for this is the formation of an ionic liquid layer resulting from the amine reacting with the odd carbon diacids, which was confirmed from laboratory synthesized 1:1 or 1:2 mixtures of the diacid:amines solutions.

Knudsen cell
“Kinetics, mechanisms and ionic liquids in the uptake of n-butylamine onto low molecular weight dicarboxylic acids”, M. Fairhurst et al., PCCP (2017) link
Another project focuses on the growth mechanism of secondary organic aerosol particles formed from the ozonolysis of a-pinene using our large volume, slow flow aerosol flow reactor. For these, three gas phase semi-volatile organic nitrates were chosen as molecular tracers. Off-line ATR-FTIR measurements coupled with real-time HR-ToF-AMS provide insights into uptake coefficients as well as gas-to-particle partition coefficients for these organic nitrates into the SOA. Additionally, the phase state of SOA particles, which governs the mechanism by which they grow in the atmosphere, is also examined.

“Understanding interactions of organic nitrates with the surface and bulk of organic films: implications for particle growth in the atmosphere”, A. Vander Wall et al., Environ. Sci. Processes Impacts (2018) link

“Evidence for a kinetically controlled burying mechanism for growth of high viscosity secondary organic aerosol”, A. Vander Wall et al., Environ. Sci. Processes Impacts (2020) link
“Enhanced gas uptake during a-pinene ozonolysis points to a burying mechanism”, A. Vander Wall et al. (2020) link
3 – “Probing Mechanisms of New Particle Formation and Growth: A Combined Experimental, Theoretical and Modeling Approach”
Using custom flow reactors we study the reaction of atmospherically relevant gases and how they lead to the formation of new particles. One system that we are currently studying involves the reaction between methanesulfonic acid (MSA) + amines + water and the influence of additional species (oxalic acid, NH3, etc.). Using state-of-the art DFT quantum calculations and mass spectrometry, we are investigating the birth and growth of sub-20 nm particles.

Particle formation and growth from oxalic acid, methanesulfonic acid, trimethylamine and water: A combined experimental and theoretical study”, K. D. Arquero et al., EST (2017) link

“Nanoparticles growth from methanesulfonic acid and methylamine: microscopic structures and formation mechanism”, J. Xu et al. Phys. Chem. Chem. Phys. (2017) link
“Uptake of water by an acid-base nanoparticle: theoretical and experimental studies of the methanesulfonic acid-methylamine system”, J. Xu et al. Phys. Chem. Chem. Phys. (2018) link
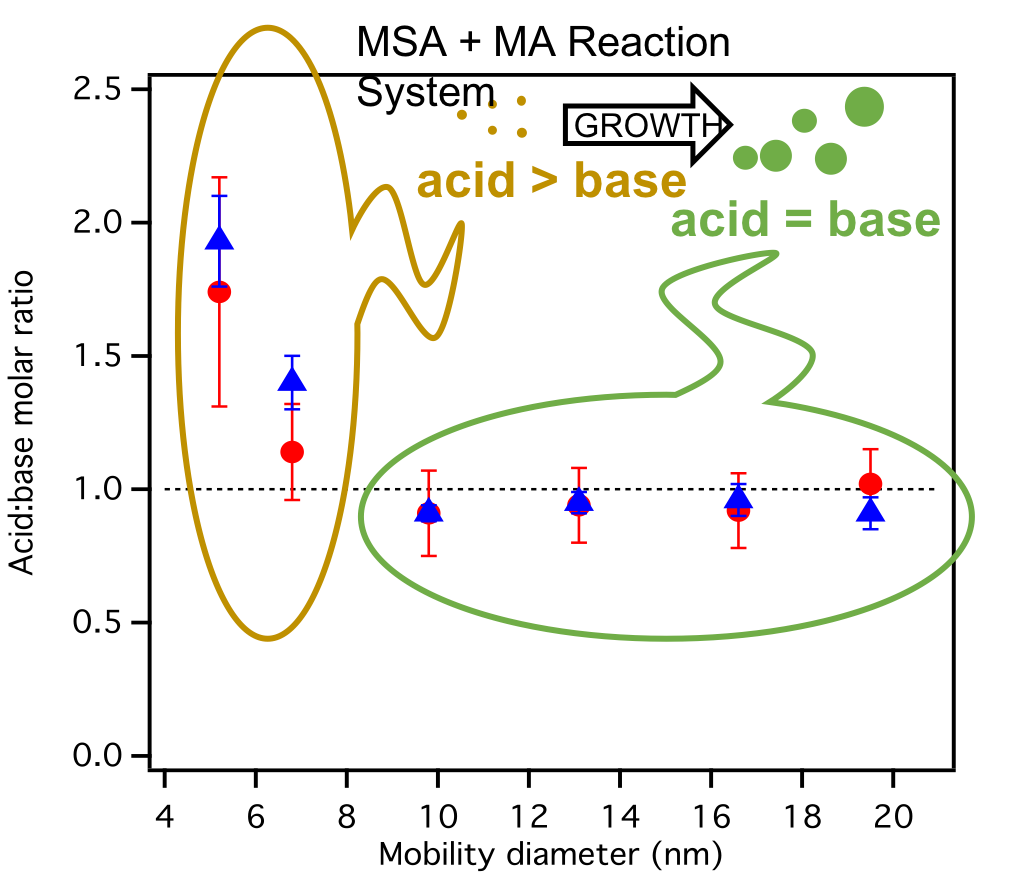
“Integrated experimental and theoretical approach to probe the synergistic effect of ammonia in methanesulfonic acid reactions with small alkylamines”, V. Perraud et al. Environ. Sci. Processes Impacts (2020) link
“Size-resolved chemical composition of sub-20 nm particles from methanesulfonic acid reactions with methylamine and ammonia”, V. Perraud, X. Li et al. ACS Earth and Space Chem. (2020) link
4 – “SusCHEM: Atmospheric Fates of Neonicotinoids and Their Role in Food Security and Agricultural Sustainability”
Neonicotinoid are a relatively new class of pesticides and represent one-third of the total world insecticide markets. They have been proposed to be at least in part responsible for bee colony collapse syndrome, and as a result restrictions are in place in Europe on some of these compounds (imidacloprid for example). While there some some understanding of their chemistry and photochemistry in aqueous solutions, relatively little is known about their reactions on solid substrates such as seeds. Neonicotinoids fall into three classes, including nitroguanidines (imidacloprid, thiamethoxam, clothianidin, dinotefuran), cyanamidines (thiacloprid, acetamiprid) and nitromethylenes (nitenpyram). This project seeks to study the photochemistry of these compounds on solid substrates using attenuated total reflectance FTIR, DART-MS and ESI-MS.

“Photochemistry of thin solid films of the neonicotinoid imidacloprid on surfaces”, K. Z. Aregahegn et al., EST (2017) link
“Photochemistry of solid films of the neonicotinoid nitenpyram”, K. Z. Aregahegn et al. EST (2018) link
“Quantum yields and N2O formation from photolysis of solid films of neonicotinoids”, W. Wang et al., J. Agric. Food Chem. (2019) link

“Experimental and theoretical studies of the environmental sensitivity of the absorption spectra and photochemistry of nitenpyram and analogs”, M. J. Ezell et al. ACS Earth Space Chem. (2019) link

“Unexpected formation of oxygen-free products and nitrous acid from the ozonolysis of the neonicotinoid nitenpyram”, W. Wang et al., Proc. Natl. Acad. Sci. USA (2020) link
