Graduate Students
Noel Salvador (Biological Chemistry graduate student)
 Melanoma cell lines commonly have a mutation in the B-RAF protein, a MAPK pathway component, that results in tumor proliferation and sustained apoptosis inhibition. CDC42 is a GTPase that activates Pak1 to exogenously phosphorylate cell division regulator proteins such as RAF and MEK in the MAPK pathway. Preventing this CDC42-Pak1 crosstalk could lead to novel cancer treatments; therefore, in collaboration with Dr. Marco De Vivo’s lab at IIT, we have developed a lead drug that inhibits CDC42 activity. My current work involves testing the effectiveness of our lead drug in both drug-resistant and non-resistant Melanoma cell lines. I am further involved in testing our lead drug in combination with other melanoma pathway inhibitors to measure any synergistic effect that can be used to improve melanoma treatment. By testing our lead drug in these manners, my goal is to optimize treatment conditions to best inhibit Melanoma and prevent Melanoma resistance.
Melanoma cell lines commonly have a mutation in the B-RAF protein, a MAPK pathway component, that results in tumor proliferation and sustained apoptosis inhibition. CDC42 is a GTPase that activates Pak1 to exogenously phosphorylate cell division regulator proteins such as RAF and MEK in the MAPK pathway. Preventing this CDC42-Pak1 crosstalk could lead to novel cancer treatments; therefore, in collaboration with Dr. Marco De Vivo’s lab at IIT, we have developed a lead drug that inhibits CDC42 activity. My current work involves testing the effectiveness of our lead drug in both drug-resistant and non-resistant Melanoma cell lines. I am further involved in testing our lead drug in combination with other melanoma pathway inhibitors to measure any synergistic effect that can be used to improve melanoma treatment. By testing our lead drug in these manners, my goal is to optimize treatment conditions to best inhibit Melanoma and prevent Melanoma resistance.
Karen(Hui) Xiao (MCSB graduate student)
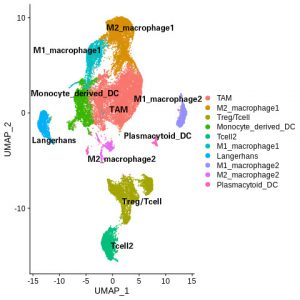
Immunosurveillance is critical in defending our body against foreign pathogens and diseases. For my project, I am interested in the role of immune system in shaping the tumor microenvironment, more specifically, how the different immune populations, such as the t-cells, the macrophages, contribute to melanogenesis. In my research, I hope to answer these questions using various approaches such as mouse models and Bioinformatics.
Postdocs and Specialists
Hira Rizwi, M.D. (Associate Specialist) Melanoma is the deadliest form of skin cancer, with a greater number of Hispanic patients presenting at a younger age and at a later stage. My project focuses on trying to understand the different cellular and epidemiologic drivers in disease initiation and progression in the Hispanic population. This would help to; 1) improve patient outcomes; 2) allow for a creation of an advanced imaging and screening tool that would assist with detection/intervention of melanomas in earlier stages, thereby reducing the financial burden of disease and increasing quality of life and survival rates. I also work closely with Vitiligo patients studying how different demographic & epidemiological factors can affect treatment response in novel therapies.
Melanoma is the deadliest form of skin cancer, with a greater number of Hispanic patients presenting at a younger age and at a later stage. My project focuses on trying to understand the different cellular and epidemiologic drivers in disease initiation and progression in the Hispanic population. This would help to; 1) improve patient outcomes; 2) allow for a creation of an advanced imaging and screening tool that would assist with detection/intervention of melanomas in earlier stages, thereby reducing the financial burden of disease and increasing quality of life and survival rates. I also work closely with Vitiligo patients studying how different demographic & epidemiological factors can affect treatment response in novel therapies.
Kathryn(Katie) Hinchee-Rodriguez , M.D., Ph.D (Post-doc)
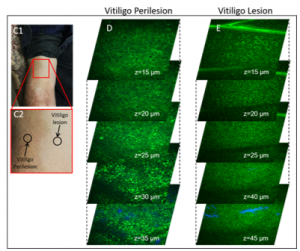
Vitiligo is an autoimmune skin disease characterized by the progressive depigmentation of epithelia initiated by CD8+ T cell destruction of melanocytes. The persistence of white patches in stable disease is poorly understood, even though melanocyte stem cells are usually present in adjacent hair follicles. Recent studies from our lab show a subpopulation of stress keratinocytes persistent in stable vitiligo. Stress keratinocytes shift their metabolism towards oxidative phosphorylation and revert their metabolism back to that of non-lesional skin when a lesion repigments, suggesting a role for lesional keratinocytes in melanocyte repopulation in vitiligo. Keratinocyte-melanocyte communication is also altered in lesional skin. I aim to identify keratinocyte-melanocyte attractive and repellant cues and develop a model to study melanocyte homeostasis in vitro, which will: 1) provide a system to study how tissue interactions influence melanocyte homeostasis; 2) lead to testing of how drugs targeting these cues can affect repigmentation in stable vitiligo.
Chi-Fen Chen, M.S. (Specialist)
One of our tasks is to understand how precancerous lesions (nevi) and skin cancer (melanoma) develop. Both nevi and melanoma originate from melanocytes, which are the cells responsible for producing pigment (melanin) in the skin. Over 80% of nevi and melanoma initiate due to an activating mutation in the BRAF (V600E) oncogene which leads to rapid proliferation of melanocytes. Activating BRAF mutations alone only produces benign nevi (common “moles”). Unlike cells that comprise melanoma and continuously proliferate, nevus cells undergo a short period of proliferation and then arrest.
My main project is studying the development of melanocytes and how they can lead to nevi (known as skin moles) and melanoma in the skin. I used BRAF mouse models which genetically alter melanocytes to answer fundamental questions about melanocyte development and tumor formation.
Jessica Shiu, M.D., Ph.D (Assistant Professor)
I am a physician scientist who has trained in immunology and clinical dermatology. I am interested in how immune cells interact with other tissue cells in melanocyte-related conditions ranging from vitiligo to nevi (commonly known as moles) and melanoma development. In vitiligo, I am studying how keratinocytes can affect immune recognition of melanocytes and the repigmentation process. I am also trying to understand how myeloid cells affect nevi growth and how this changes in melanoma. I work with a team of bioengineers, bioinformaticians, molecular biologists and mathematicians to help understand these processes in animal models and humans.
Linh Vuong, Ph.D (Assistant Project Scientist)
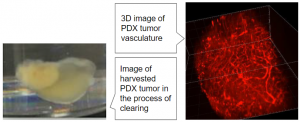
Cancer cells initially utilize tissue-resident vessels for nutrients, oxygen, and waste removal. Eventually cancer cells recruit endothelial cells (ECs) and pericytes to form new blood vessels from pre-existing vessels. Tumor angiogenesis allows the tumor to grow and metastasize. RHOJ, a member of the subfamily of small Rho GTPases, has been shown to control both tumor growth (Ruiz R et al., 2017) and tumor angiogenesis (Wilson E et al., 2014). RHOJ is primarily expressed in ECs and in some human tumor types (i.e. gastric cancer and melanoma) (Kaur S et al., 2011; Kim C et al., 2016; Ruiz R et al., 2017). Tumors with high RHOJ expression in patients had increased lymphovascular invasion (Kim C et al., 2014).
My research focus is aimed at elucidating the molecular function of RHOJ in cancer cells and ECs. The approach is to block the activity of RHOJ by interfering with the RHOJ-PAK1 interaction. Lost of this interaction inhibits downstream signaling cascades that regulate anti-apoptosis, pro-proliferation, and decrease contractility and permeability, which are key hallmarks of tumor progression (Radu M et al., 2014). We will use a combination of mouse model and cancer cell systems and apply biochemical assays and high-throughput sequencing analyses to examine the role of RHOJ-PAK1 signaling in tumor progression. This study will provide insight into the function of RHOJ and molecular cross-talk between tumor cells and ECs that allows tumor to progress.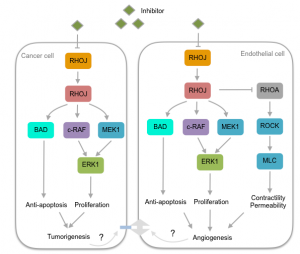
Schematic of RHOJ-PAK1 signaling pathway in cancer and endothelial cells. Adopted and modified from Radu M et al., 2014 and Ye DZ and Field J 2012. We will use an inhibitor to block RHOJ-PAK1 interaction to investigate how the pathway is disrupted. The goal is to decipher the role of RHOJ in cancer and endothelial cells during tumor progression.
Undergraduates
Rachel Pham (Major: Biology)
Vance Ku (Major: Bioengineering)
Emily Pham
Other Open Positions-contact Dr. Ganesan
Alumni
Sahil Telang – Medical Student at USC
Anastasia Chahine – Internal Medicine Resident at UC Irvine
Terry Nguyen – Medical Laboratory Scientist Lead at Natera
Pezhman Mobasher – Internal Medicine Resident at Saint Agnes Medical Center
Sohail Jahid – Scientist at Avita
Jessica Flesher, Ph.D. – Post-doc Research Fellow at Massachusetts General Hospital
Madeline McCanne (Orange County School of Arts High School)
Priya Vasudeva – Medical Student at Campbell University School of Osteopathic Medicine
Rolando Ruiz, Ph.D. – Senior Scientist at Quest diagnostics
Marc Liggins – Postdoc at UCSD
Francisco Espitia – Field Application Scientist at Beckman Coulter
Elyse Paterson, Ph.D. – EHS Specialist at OSHU
Ismar Rodriguez (UCSD)
Shyam Chandrasekar (UCSD)
Hannah Mumm (Occidental College Undergraduate)
Jair Fabian (Orange Coast Community College) – Undergraduate at UCLA
Jennifer Sui (Undergraduate at Vanderbilt)
Katerina Yale (MD Student)
Natalie Chico (HS student-Summer 2014)
Shawon Debnath (Postdoc)
Ankita Shukla – Ph.D. candidate at UCI.
Cameron Shaaban, M.S. – Works at Baxter International.
Sam Sandhu (Kettering College PA Studies)
Sara Carmona – Ph.D. candidate at UCI in Amy Sun Lab.
Philip Newton (UCLA)
Amy Sun (Summer Student)
Jonathan Schilling – Medical Student at UCI Medical School
Cammy Malhis (Touro College of Osteopathic Medicine – NY)
Safoora Ahmed (Undergraduate)
Amy Hopkin, Ph.D. – Principal Scientist at AVITA Medical
Hsiang Ho, Ph.D. – Scientist at Allergan
Rubina
For more information on any of the ongoing projects please refer to Research Projects Tab located in the Navigation Bar above.