Mini-course (5 learning modules) offered by
Graduate Professional Success in STEM (GPS-STEM) at UC Irvine
Date/Time: August 14 (Fridays); 12:00PM (PST)
Duration: 5 Weeks [August 14, 2020 – Sept 11, 2020]
Currently, a significant number of biomedical PhDs are employed in scientific industry and the demand for highly skilled PhD level scientists keeps growing. Biomedical science PhDs can transition into ~20 different non-academic careers in the private sector, government and nonprofits. Out of the wide variety of available industry jobs, medical writing jobs are becoming increasingly popular amongst biomedical PhDs.
Historically, the medical writing field has employed a large number of professional degree holders e.g PharmD and MDs; however, lately this trend has shifted towards hiring basic science PhDs. Healthcare industries value biomedical science PhDs due to their vast subject matter expertise, ability to understand pathophysiology of different disease conditions, drug mechanisms, high level of analytical skills, and exceptional writing traits. However, there is inadequate knowledge amongst trainees (PhD students & Postdocs) on medical writing as a plausible future career as well as mechanisms of transitioning into these roles.
In order to fill the gap in knowledge and experience related to medical writing careers, GPS-STEM at UC Irvine is offering a mini-course, designed to help trainees learn the landscape of the medical writing field, roles and responsibilities of a medical writer, and the variety of transferable skills required to transition into these roles.
Mission: Help trainees understand ins-and-outs of the medical writing field & teach ways to transition successfully
Goals
- Help trainees understand similarities and differences between grant writing, manuscript writing, & medical writing
- Gain deeper understanding of medical writing field
- Learn about different kinds of medical writing e.g regulatory writing, health communications, publications, diagnostics and other kinds of educational writing
- Learn transferable skills required to transition into these roles plus skills required on the job to be successful
- Practice writing tests required during the hiring process
- Learn about steps and techniques to successfully transition into writing roles
Course Coordinators:
Acknowledgements: GPS-STEM program would like to sincerely thank William Kim for helping with the curriculum design, Huixuan Liang for bringing regulatory affairs writing expertise and all the UCI alumni for helping take this course off the ground.
Special thanks to Joanne Ly, PhD student in Biomedical Engineering & Multimedia Director of GPS-STEM program, for beautiful graphics.
Course Curriculum & Schedule
Module 1 [Aug 14, 2020]: Introduction & overview of the field of medical writing. Understand the landscape of medical writing field, kinds of medical writing, skills required to transition. Basics of Medical Writing
- Publications
- Regulatory
- Medical Education
- Health communication
- Laboratory diagnostics
- Promotional
- FDA – Regulatory writing
Required skill sets (Transferrable Skills) & qualifications for the role
Speakers/Panelists:
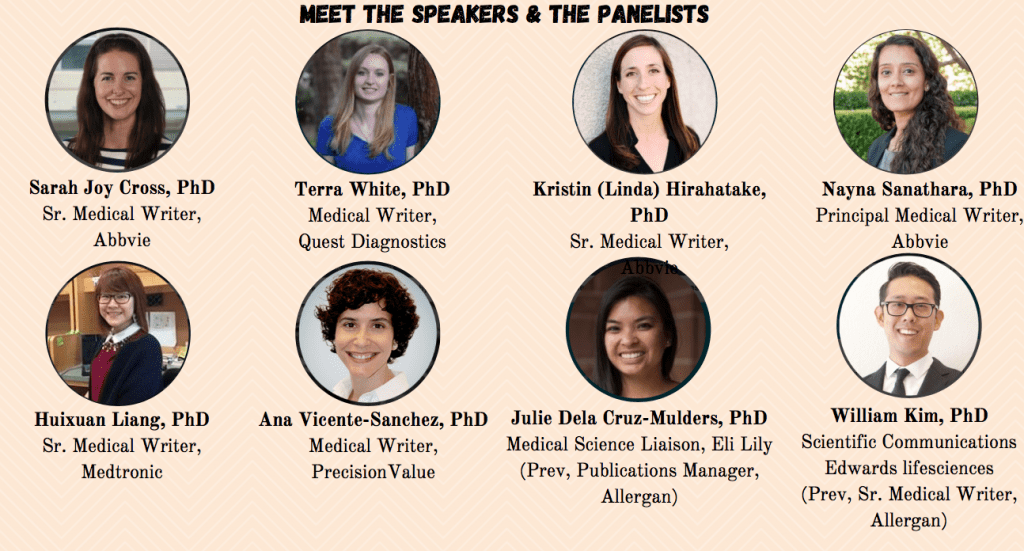
- William Kim, PhD. Manager, Scientific Communications at Edwards Lifescience (Prev. Sr. Medical Writer, Allergan)
- Julie Dela Cruz-Mulder, PhD. Medical Science Liaison, Eli Lilly. Previously, Publications Manager, Allergan (now Abbvie)
- Huixuan Liang, PhD. Sr. Medical Writer at Medtronic
- Terra White, PhD. Medical Writer at Quest Diagnostics
Module 2 [Aug 21, 2020]: Where do medical writers work/Career development/progression Learn about many places where medical writers are employed at including avenues of career development in each. Our speakers will share medical writer roles in Biotech, medical device and pharmaceutical companies, CROs, communication agencies, education sector, freelance and medical writing agencies.
Major discussion topics –
- Is there scope of lateral transition or climbing ladder
- Freelance medical writing
- Medical writing agencies
- Average time one spends in a writing job
- What other kind of roles/jobs medical writers transition into e.g is it easy to transition from medical writing to regulatory writing & vice-versa
Speakers/Panelists:
- William Kim, PhD. Manager, Scientific Communications at Edwards Lifescience (Prev. Sr. Medical Writer, Allergan)
- Julie Dela Cruz-Mulder, PhD. Medical Science Liaison, Eli Lilly. Previously, Publications Manager, Allergan (now Abbvie)
- Ana Vicente-sanchez, PhD. Scientific Associate/Medical Writer, PrecisionValue ((Medical Writing Agency)
Module 3 [Aug 28, 2020]: Examples of the types of work that medical writers perform on the job (with examples). During this session, speakers will provide examples of the kinds of writing medical writers perform. Discuss key collaborators e.g Pub Managers, R&D, Clinical teams, MDs, PhDs. and understanding the kinds of working relationships
- Journal articles
- Abstract writing
- Conference posters/presentations
- Slides/internal training materials
- Data analysis
- Project management
- Project timeline
- Stakeholder engagement
- Healthcare agencies
- Documentation
- Authorship guidelines – Differences b/w academia vs Industry
Required Skill Sets (Transferrable Skills)
Speakers/Panelists:
- Nayna Sanathara, PhD. Principal Medical Writer at Abbvie
- Sarah Joy Cross, PhD. Sr. Medical Writer at Abbvie
- Emma Flores-Kim, PhD. Scientific Affairs Manager, NeoTract
Module 4 [Sept 4, 2020]: Tests on writing and editing with examples of kinds of writing exams
- In this module we will offer publicly available tests or examples for practice to get familiar with the application process
- List out types of tests people have encountered (will most likely test ability to write and analyze/interpret data)
- Write abstract and create 5 min presentation slide based on several pages of clinical data
- Create a complex data figure on excel similar to given example and write up a paragraph describing the results in the figure
- Brush up on basic stats, eg, confidence intervals, standard deviation, p value
- Writing assignments* will be due at the end of the course for receiving certificate of course completion.
- What skill sets employers look for in medical writers (transferable skills from PhD/Postdoc to be highlighted in the cover letters)
Speakers/Panelists:
- Emma Flores-Kim, PhD. Scientific Affairs Manager, NeoTract
- Huixuan Liang, PhD. Sr. Medical Writer at Medtronic
- Sarah Joy Cross, PhD. Sr. Medical Writer at Abbvie
- Terra White, PhD. Medical Writer at Quest Diagnostics
Module 5 [Sept 11, 2020] : How to transition into medical writing roles – Panel discussion & e-Networking.
- Examples of Resume, cover letter tailoring tricks for these jobs
- Effective use of LinkedIn for job search in the field
- Networking events & Alumni mentoring programs
Medical writers in attendance –
- Emma Flores-Kim, PhD. Scientific Affairs Manager, NeoTract
- Huixuan Liang, PhD. Sr. Medical Writer at Medtronic
- Sarah Joy Cross, PhD. Sr. Medical Writer at Abbvie
- Terra White, PhD. Medical Writer at Quest Diagnostics
- Kristin Hirahatake, PhD. Sr. Medical Writer at Abbvie
- Nayna Sanathara, PhD. Principal Medical Writer at Abbvie
- Ana Vicente-sanchez, PhD. Scientific Associate/Medical Writer, PrecisionValue ((Medical Writing Agency)
- William Kim, PhD. Manager, Scientific Communications at Edwards Lifescience (Prev. Sr. Medical Writer, Allergan)
- Julie Dela Cruz-Mulder, PhD. Medical Science Liaison, Eli Lilly. Previously, Publications Manager, Allergan (now Abbvie)
*Writing Assignments Due [Sept 14, 2020]: Submit assignments, receive feedback from speakers/panelists – Certificate of course completion
Resources for Medical Writing Careers:
- How to prepare for a medical writing career as a graduate student or postdoc
- The medical writing career path for PhDs
- Is a career in medical writing right for you?
- Genetics PhD to medical writer and entrepreneur
- So you want to be a medical writer – Interview
- What types of medical writing are there? AMWA
- A career in medical communication: Steps to success
- An ultimate guide to becoming a medical writer
- 5 Steps to a successful job search in medical writing
- How to prepare for a medical writing career as a graduate student or postdoc