(73) “A Nickel-Catalyzed Cross-Electrophile Coupling Reaction of 1,3-Dimesylates for Alkylcyclopropane Synthesis: Investigation of Stereochemical Outcomes and Radical Lifetimes” Chen, P.-P.; McGinnis, T. M.; Lin, P. C.; Hong, X.*; Jarvo, E. R.* ACS Catal. 2023, 13, 5472–5481.
DOI: https://doi.org/10.1021/acscatal.3c00905

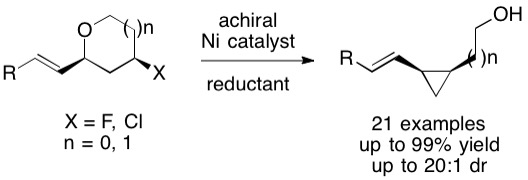
(72) “Stereospecific Nickel-Catalyzed Cross-Electrophile Coupling Reaction of Alkyl Mesylates and Allylic Difluorides to Access Enantioenriched Vinyl Fluoride-Substituted Cyclopropanes” Lin, P. C.; Joshi, C.; McGinnis, T. M.; Chandra Mallojjala, S.; Sanford, A. B.; Hirschi, J. S.*; Jarvo, E. R.* ACS Catal. 2023, 13, 7, 4488–4499.
DOI: https://doi.org/10.1021/acscatal.3c00257

(71) “Industrial Applications of Transition Metal-Catalyzed Asymmetric Cross-Coupling Reactions (C–C and C–N Bond Formations)” Lin, P. C.; Jarvo, E. R.* Comprehensive Chirality 2nd edition. 2023.
DOI: https://doi.org/10.1016/B978-0-32-390644-9.00031-7
(70) “Halogenation Reactions of Alkyl Alcohols Employing Methyl Grignard Reagents” Hirbawi, N.; Lin, P. C.; Jarvo, E. R.* J. Org. Chem. 2022, 87, 12352–12369.
DOI: https://doi.org/10.1021/acs.joc.2c01590
Highlighted in Organic Chemistry Portal: https://www.organic-chemistry.org/abstracts/lit8/640.shtm

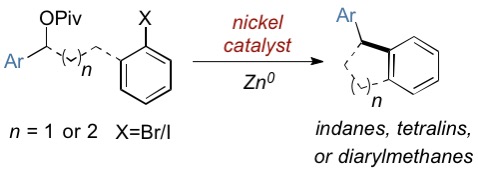
(69) “Synthesis of Vicinal Carbocycles by Intramolecular Nickel-Catalyzed Conjunctive Cross-Electrophile Coupling Reaction” Hewitt, K. A.; Herbert, C. A.; Jarvo, E. R.* Org. Lett. 2022, 24, 6093–6098.
DOI: https://doi.org/10.1021/acs.orglett.2c02481

(68) “Zinc-Mediated Transformation of 1,3-Diols to Cyclopropanes for Late-Stage Modification of Natural Products and Medicinal Agents” McGinnis, T. M.; Thane, T. A.; Jarvo, E. R.* Org. Lett. 2022, 24, 5619–5623.
DOI: https://doi.org/10.1021/acs.orglett.2c02362

(67) “Ligand-Based Control of Nickel Catalysts: Switching Chemoselectivity from One-Electron to Two-Electron Pathways in Competing Reactions of 4-Halotetrahydropyrans” Thane, T. A.; Jarvo, E. R.* Org. Lett. 2022, 24, 5003–5008.
DOI: https://doi.org/10.1021/acs.orglett.2c01335

Invited cover art: https://pubs.acs.org/toc/orlef7/24/28
(66) “C–C Bond Formation Through Cross-Electrophile Coupling Reactions” Hewitt, K. A.; Lin, P. C.; Raffman, E. T. A.; Jarvo, E. R.* Comprehensive Organometallic Chemistry IV. 2022, 12, 89–119.
DOI: https://doi.org/10.1016/B978-0-12-820206-7.00092-5
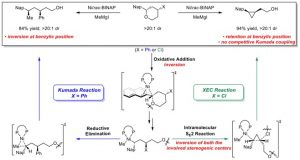
(65) “Nickel-Catalyzed Domino Cross-Electrophile Coupling Dicarbofunctionalization Reaction to Afford Vinylcyclopropanes” Hewitt, K. A.; Xie, P.-P.; Thane, T. A.; Hirbawi, N.; Zhang, S.-Q.; Matus, A. C.; Lucas, E. L.; Hong, X.*; Jarvo, E. R.* ACS Catal. 2021, 11, 14369–14380.
DOI: https://doi.org/10.1021/acscatal.1c04235

(64) “Nickel-Catalyzed Kumada Cross-Coupling Reactions of Benzylic Sulfonamides” Hewitt, K. A.; Herbert, C. A.; Matus, A. C.; Jarvo, E. R.* Molecules, 2021, 26(19), 5947.
DOI: https://doi.org/10.3390/molecules26195947
A part of the special issue: A Tribute to Design and Strategy in Organic Synthesis in Honor of Stephen Hanessian
(63) “Nickel-Catalyzed Cross-Electrophile Coupling of the Difluoromethyl Group for Fluorinated Cyclopropane Synthesis” Lucas, E. L.; McGinnis, T. M.; Castro, A. J.; Jarvo, E. R.* Synlett, 2021, 32, 1525–1530.
Highlighted in Thieme Chemistry News: https://www.thieme.de/en/thieme-chemistry/synform-news-novel-entry-to-fluorinated-cyclopropanes-163476.htm
Selected as Synlett Best Paper Award 2021!
https://www.thieme.de/en/thieme-chemistry/2021-winners-167159.htm

(62) “Harnessing C–O Bonds in Stereoselective Cross-Coupling and Cross-Electrophile Coupling Reactions” Sanford, A. B.; Jarvo, E. R.* Synlett 2021, 32, 1151–1156.
Invited Account of our research.
DOI: http://10.1055/s-0040-1705987
Selected for the journal cover.
(61) “Nickel-Catalyzed Alkyl–Alkyl Cross-Electrophile Coupling Reaction of 1,3-Dimesylates for the Synthesis of Alkylcyclopropanes” Sanford, A. B.; Thane, T. A.; McGinnis, T. M.; Chen, P.-P.; Hong, X.; Jarvo, E. R. J. Am. Chem. Soc. 2020, 142, 5017–5023.
DOI: https://doi.org/10.1021/jacs.0c01330
(60) “Engaging Sulfonamides: Intramolecular Cross-Electrophile Coupling Reaction of Sulfonamides with Alkyl Chlorides” Lucas, E. L.; Hewitt, K. A.; Chen, P.-P.; Castro, A. J.; Hong, X.; Jarvo, E. R. J. Org. Chem. 2020, 85, 1775-1793.
Selected as a Featured Article by the editor.
DOI: https://doi.org/10.1021/acs.joc.9b02603
JOC Issue Cover:
Selected as Journal of Organic Chemistry’s 2021 Outstanding Article of the Year Award, highlighted in ACS Axial:
https://axial.acs.org/2021/03/29/meet-the-journal-of-organic-chemistrys-2021-outstanding-article-of-the-year-award-recipients/
(59) “Identification of the Active Catalyst for Nickel-Catalyzed Stereospecific Kumada Coupling Reactions of Ethers” Dawson, D. D.; Oswald, V. F.; Borovik, A. S.; Jarvo, E. R. Chem. Eur. J. 2020, 26, 3044–3048.
https://onlinelibrary.wiley.com/doi/full/10.1002/chem.202000215
(58) “Stereospecific Cross-Coupling Reactions Provide Conformationally-Biased Arylalkanes with Anti-Leukemia Activity” Sanford, A. B.; Tollefson, E. J.; Jarvo, E. R. Isr. J. Chem. 2020, 60, 402–405.
Invited manuscript for special issue commemorating Wolf Prize of Professors Buchwald and Hartwig
https://doi.org/10.1002/ijch.201900071
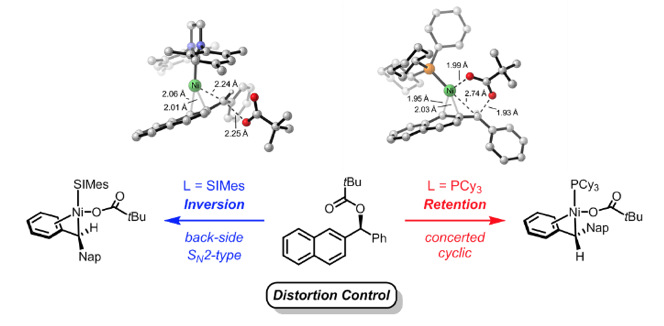
(57) “A Unified Explanation for Chemoselectivity and Stereospecificity of Ni-Catalyzed Kumada and Cross-Electrophile Coupling Reactions of Benzylic Ethers: A Combined Computational and Experimental Study” Chen, P.-P.*; Lucas, E. L.*; Greene, M. A.; Zhang, S.; Tollefson, E. J.; Erickson, L. W.; Taylor, B. L.; Jarvo, E. R.; Hong, X. J. Am. Chem. Soc. 2019, 141, 5835–5855.
*These authors contributed equally
https://pubs.acs.org/doi/abs/10.1021/jacs.9b00097
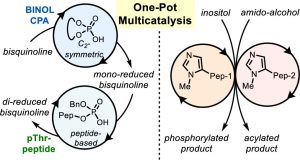
(56) “Outer-Sphere Control for Divergent Multicatalysis with Common Catalytic Moieties” Shugrue, C. R.; Sculimbrene, B. R.; Jarvo, E. R.; Mercado, B. Q.; Miller, S. J. J. Org. Chem. 2019, 84, 1664–1672. DOI: 10.1021/acs.joc.8b03068
(55) “Nickel-Catalyzed Directed Hydroarylation of Alkynes with Boronic Acids” Hanna, L. E.; Konev, M.; Jarvo, E. R. Eur J. Org. Chem. 2019, 184–187. DOI: 10.1002/ejoc.201801494
(54) “Keeping Track of Electrons” Lucas, E. L.; Jarvo, E. R. Acc. Chem. Res. 2018, 51, 567–572. DOI: 10.1021/acs.accounts.7b00432 (53) “Nickel-Catalyzed Hydrogenolysis and Conjugate Addition of 2-(Hydroxymethyl)pyridines via Organozinc Intermediates” Hanna, L. E.; Harris, M. R.; Domon, K.; Jarvo, E. R. Org. Lett. 2017, 19, 6304–6307.
(53) “Nickel-Catalyzed Hydrogenolysis and Conjugate Addition of 2-(Hydroxymethyl)pyridines via Organozinc Intermediates” Hanna, L. E.; Harris, M. R.; Domon, K.; Jarvo, E. R. Org. Lett. 2017, 19, 6304–6307.
DOI: 10.1021/acs.orglett.7b03049

(52) “Mechanism and Origins of Ligand-Controlled Stereoselectivity of Ni-Catalyzed Suzuki–Miyaura Coupling with Benzylic Esters: A Computational Study.” Zhang, S.; Taylor, B. L. H.; Ji, C.; Gao, Y.; Harris, M. R.; Hanna, L. E.; Jarvo, E. R.; Houk, K. N.; Hong, X. J. Am. Chem. Soc. 2017, 139, 12994–13005.
DOI: 10.1021/jacs.7b04973
(51) “Stereospecific and stereoconvergent cross-couplings between alkyl electrophiles.” Lucas, E. L.; Jarvo, E. R. Nat. Rev. Chem. 2017, 1, 0065.
DOI: 10.1038/s41570-017-0065
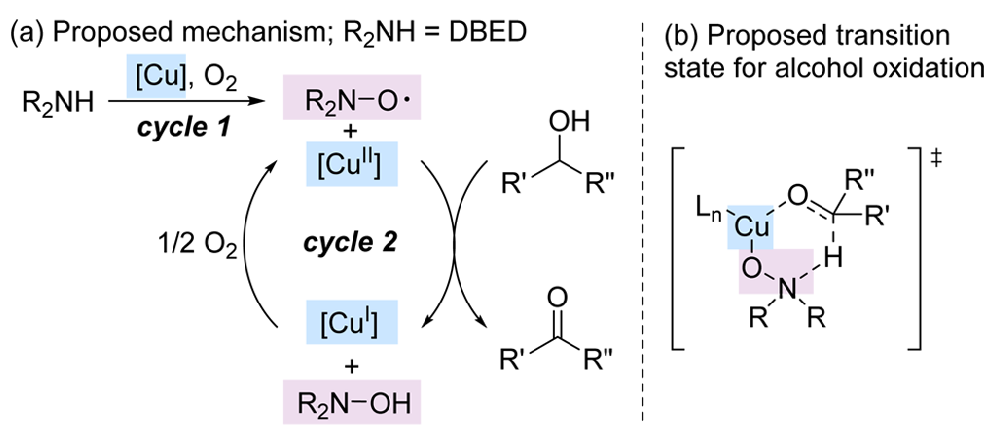
(50) “Nitroxyl Surprise: A Simple Amine Additive Revealed as Copper’s Co-Catalyst in the Aerobic Oxidation of Alcohols” Konev, M. O.; Jarvo, E. R. ACS Cent. Sci. 2017, 3, 272–274.
DOI: 10.1021/acscentsci.7b00138
(49) “Nickel-Catalyzed Cross-Electrophile Coupling of Alkyl Fluorides: Stereospecific Synthesis of Vinylcyclopropanes” Erickson, L. W.; Lucas, E. L.; Tollefson, E. J.; Jarvo, E. R. J. Am. Chem. Soc. 2016, 138, 14006–14011.
DOI: 10.1021/jacs.6b07567
Highlighted in Synfacts 2017, 13, 63.
DOI:10.1055/s-0036-1589748
(48) “Decarboxylative Alkyl–Alkyl Cross-Coupling Reactions” Konev, M. O.; Jarvo, E. R. Angew. Chem. Int. Ed. 2016, 55, 11340–11342.
DOI: http://onlinelibrary.wiley.com/doi/10.1002/anie.201605593/abstract
(47) “Intra- and Intermolecular Nickel-Catalyzed Reductive Cross-Electrophile Coupling Reactions of Benzylic Esters with Aryl Halides” Konev, M. O.; Hanna, L. E.; Jarvo, E. R. Angew. Chem. Int. Ed. 2016, 55, 6730–6733.
Highlighted in Org. Process Res. Dev. 2016, 20, 1109−1117. DOI: http://pubs.acs.org/doi/pdf/10.1021/acs.oprd.6b00218
(46) “Selective Cross-Electrophile Coupling Using Dual Catalysis” Hanna, L. E.; Jarvo, E. R. Angew. Chem. Int. Ed. 2015, 54, 15618. doi/10.1002/anie.201509444
Invited highlight.
(45) “Silver-Catalyzed Enantioselective Propargylation Reactions of N-Sulfonylketimines.” Osborne, C. A.; Endean, T. B. D.; Jarvo, E. R. Org. Lett. 2015, 17, 5340. DOI 10.1021/acs.orglett.5b02692
Highlighted in Synfacts 2016, 12, 14.
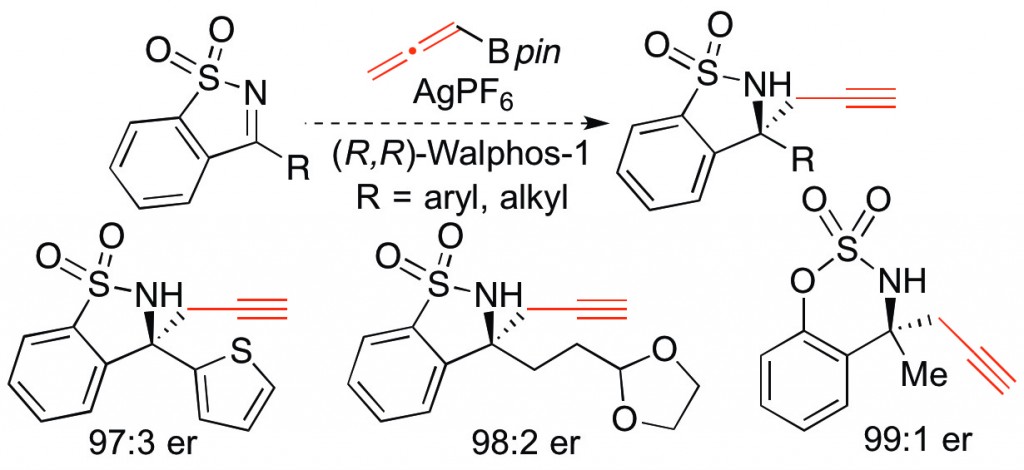 (44) “Stereospecific Nickel-Catalyzed Cross-Coupling Reactions of Benzylic Ethers with Isotopically-Labeled Grignard Reagents.” Dawson, D. D.; Jarvo, E. R. Org. Proc. Res. Dev. 2015, 19, 1356. DOI: 10.1021/acs.oprd.5b00148
(44) “Stereospecific Nickel-Catalyzed Cross-Coupling Reactions of Benzylic Ethers with Isotopically-Labeled Grignard Reagents.” Dawson, D. D.; Jarvo, E. R. Org. Proc. Res. Dev. 2015, 19, 1356. DOI: 10.1021/acs.oprd.5b00148
Invited contribution to special issue “Non-precious Metal Catalysis”
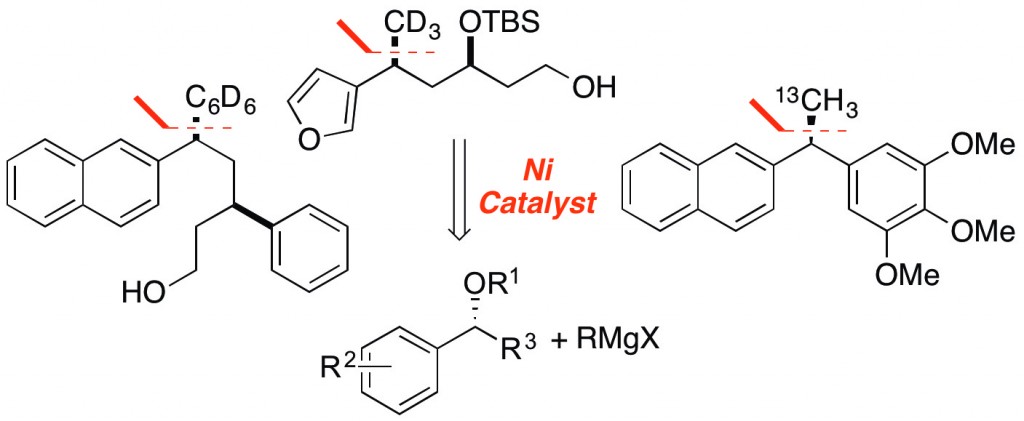 (43) “Stereospecific Intramolecular Reductive Cross-Electrophile Coupling Reactions for Cyclopropane Synthesis. Tollefson, E. J.; Erickson, L. W. Jarvo, E. R. J. Am. Chem. Soc. 2015, 137, 9760. DOI:10.1021/jacs.5b03870
(43) “Stereospecific Intramolecular Reductive Cross-Electrophile Coupling Reactions for Cyclopropane Synthesis. Tollefson, E. J.; Erickson, L. W. Jarvo, E. R. J. Am. Chem. Soc. 2015, 137, 9760. DOI:10.1021/jacs.5b03870
Highlighted in Org. Proc. Res. Dev. 2016, 20, 105.
DOI: 10.1021/acs.oprd.6b00012

(42) “Stereospecific Nickel-Catalyzed Cross-Coupling Reactions of Benzylic Ethers and Esters.” Tollefson, E. J.; Hanna, L. E.; Jarvo, E. R. Acc. Chem. Res. 2015, 48, 2344. Link
Invited contribution to special issue, “Earth Abundant Metals in Homogeneous Catalysis”
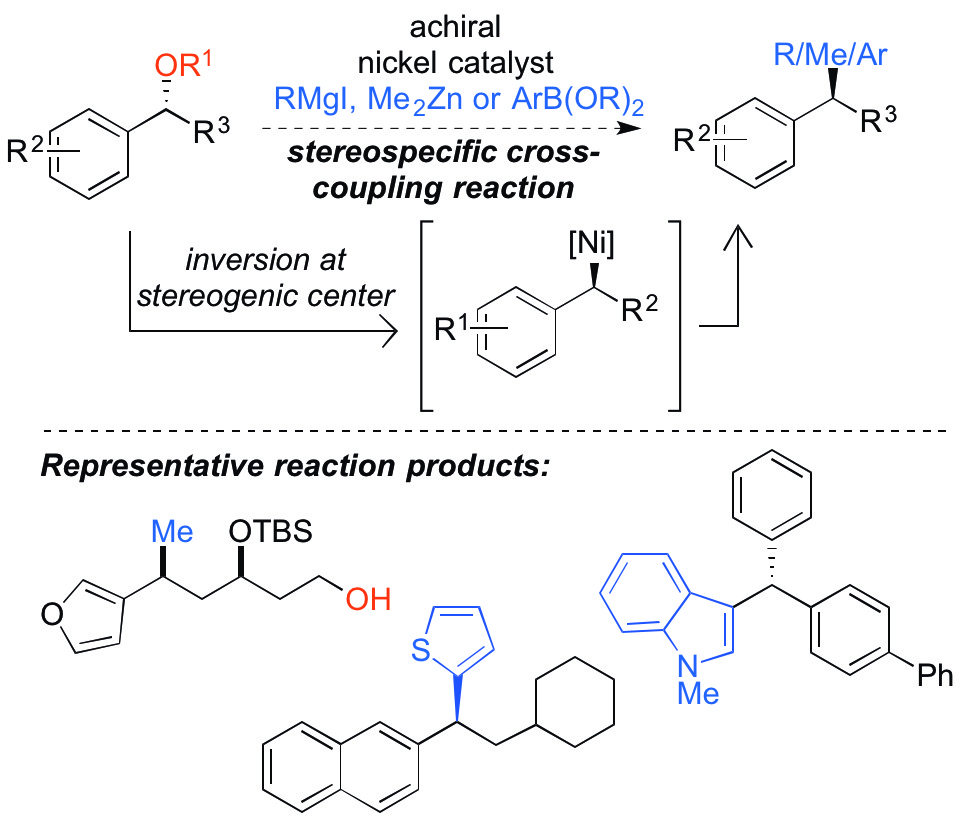
(41) “Selective synthesis of either enantiomer of an anti-breast cancer agent via a common enantioenriched intermediate.” Johnson, A. G.; Tranquilli, M. M.; Harris, M. R.; Jarvo, E. R. Tetrahedron Lett. 2015, 56, 3486. Link
Invited Manuscript for Symposium-In-Print in Memory of Professor Wasserman
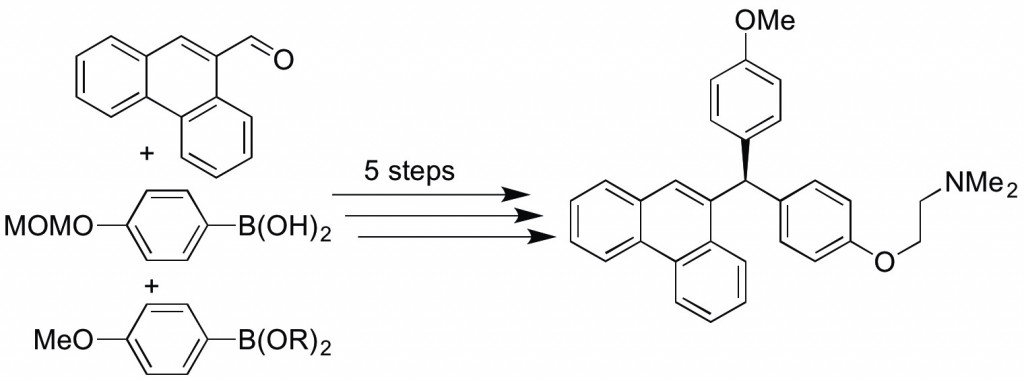
(40) “Stereospecific Cross-Coupling Reactions of Aryl-Substituted Tetrahydrofurans, Tetrahydropyrans, and Lactones.” Tollefson, E. J.; Dawson, D. D.; Osborne, C. A.; Jarvo, E. R. J. Am. Chem. Soc. 2014, 136, 14951. Link
Highlighted in Org. Proc. Res. Dev. 2015, 19, 322. DOI: 10.1021/acs.oprd.5b00022

(39) “Enantiospecific Intramolecular Heck Reactions of Secondary Benzylic Ethers.” Harris, M. R.; Konev, M. O.; Jarvo, E. R. J. Am. Chem. Soc. 2014, 136, 7825. Link
Highlighted in Synfacts 2014, 10, 932.
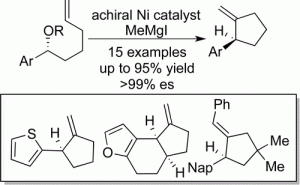
(38) “Diaryl and Heteroaryl Sulfides: Synthesis via Sulfenyl Chlorides and Evaluation as Selective Anti-Breast-Cancer Agents.” Yonova, I. M.; Osborne, C. A.; Morrissette, N. S.; Jarvo, E. R. J. Org. Chem. 2014, 79, 1947. Link
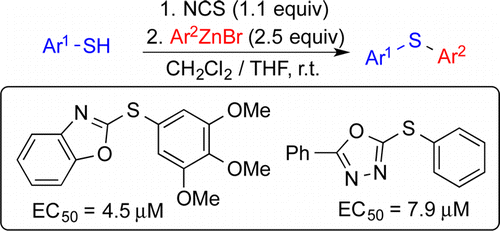
(37) “Stereospecific Nickel-Catalyzed Cross-Coupling Reactions of Alkyl Grignard Reagents and Identification of Selective Anti-Breast-Cancer Agents.” Yonova, I. M.; Johnson, A. G.; Osborne, C. A.; Moore, C. E.; Morrissette, N. S.; Jarvo, E. R. Angew. Chem. Int. Ed. 2014, 53, 2422. Link
Highlighted as “Synfact of the Month” in Synfacts 2014, 10, 515.
(36) “Enantioselective Propargylation and Allenylation Reactions of Ketones and Imines.” Wisniewska, H. M.; Jarvo, E. R. J. Org. Chem. 2013, 78, 11629. Link
(35) “Functional-Group-Tolerant, Nickel-Catalyzed Cross-Coupling Reaction for Enantioselective Construction of Tertiary Methyl-Bearing Stereocenters.” Wisniewska, H. M.; Swift, E. C.; Jarvo, E. R. J. Am. Chem. Soc. 2013, 135, 9083. Link
(34) “Asymmetric Transition Metal-Catalyzed Cross-Coupling Reactions for the Construction of Tertiary Stereocenters.” Swift, E. C.; Jarvo, E. R. Tetrahedron 2013, 69, 5799. Link
(33) “Silver-Catalyzed Allenylation and Enantioselective Propargylation Reactions of Ketones.” Kohn, B. L.; Ichiishi, N.; Jarvo, E. R. Angew. Chem. Int. Ed. 2013, 52, 4414. Link
Highlighted in Synfacts 2013, 9, 766.

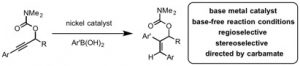
(32) “Retention or Inversion in Stereospecific Nickel-Catalyzed Cross-Coupling of Benzylic Carbamates with Arylboronic Esters: Control of Absolute Stereochemistry with an Achiral Catalyst.” Harris, M. R.; Hanna, L. E.; Greene, M. A.; Moore, C. E.; Jarvo, E. R. J. Am. Chem. Soc. 2013, 135, 3303. Link
(31) “Synthesis of Enantioenriched Triarylmethanes by Stereospecific Cross-Coupling Reactions.” Taylor, B. L. H.; Harris, M. R.; Jarvo, E. R. Angew. Chem. Int. Ed. 2012, 51, 7790. Link

(30) “Traceless Directing Group for Stereospecific Nickel-Catalyzed Alkyl−Alkyl Cross-Coupling Reactions.” Greene, M. A., Yonova, I. M., Williams, F. J., Jarvo, E. R. Org. Lett. 2012, 14, 4293. Link
Highlighted in Synfacts 2012, 8, 1228.
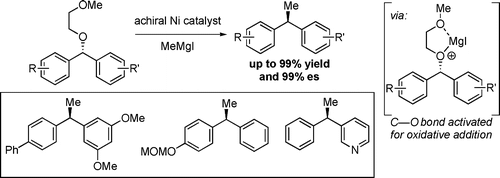
(29) “Developments in Transition-Metal Catalyzed Reactions Using Electrophilic Nitrogen Sources.” Barker, T. J., Jarvo, E. R. Synthesis, 2011, 24, 3954. Link
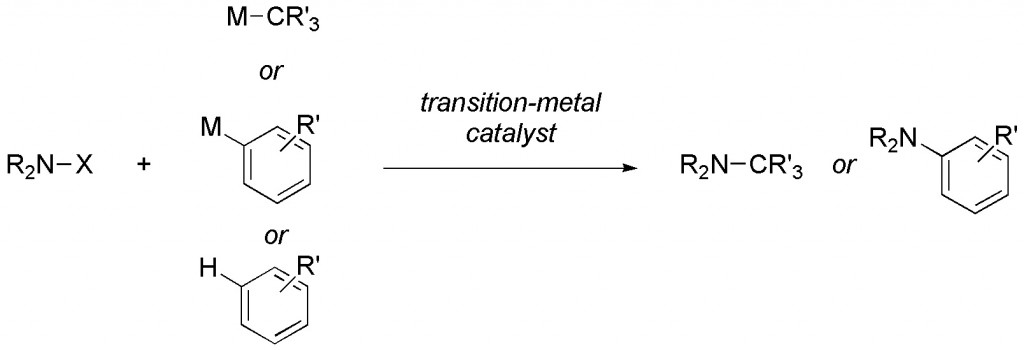 (28) “Rhodium-Catalyzed Redox Allylation Reactions of Ketones.” F. J. Williams, R. E. Grote, E. R. Jarvo. Chem. Commun. 2012, 48, 1496. Link
(28) “Rhodium-Catalyzed Redox Allylation Reactions of Ketones.” F. J. Williams, R. E. Grote, E. R. Jarvo. Chem. Commun. 2012, 48, 1496. Link
Invited manuscript for “Emerging Investigators” Issue
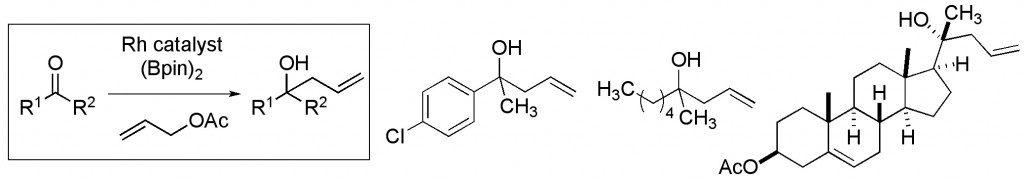
(27) “Regioselective Silver-Mediated Kondakov-Darzens Olefin Acylation.” Barczak, N. T., Jarvo, E. R. Chem.–Eur J. 2011, 17, 12912. Link
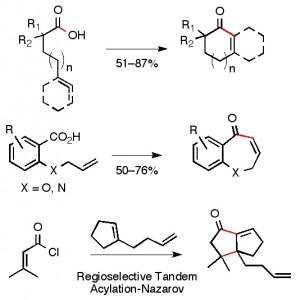
(26) “Construction of Enantioenriched Tertiary Stereogenic Centers by Nickel- and Palladium-Catalyzed Cross-Coupling Reactions of Alkyl Electrophiles.” Taylor, B. L. H., Jarvo, E. R. Synlett, 2011, 19, 2761. Link
Invited highlight of Publication #19
(25) “Palladium-Catalyzed Annulation Reactions for Diastereoselective Cyclopentene Synthesis.” B. L. Kohn, E. R. Jarvo. Org. Lett. 2011, 13, 4858. Link
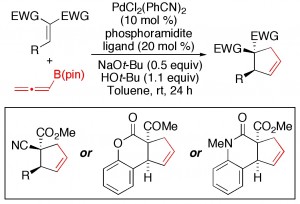
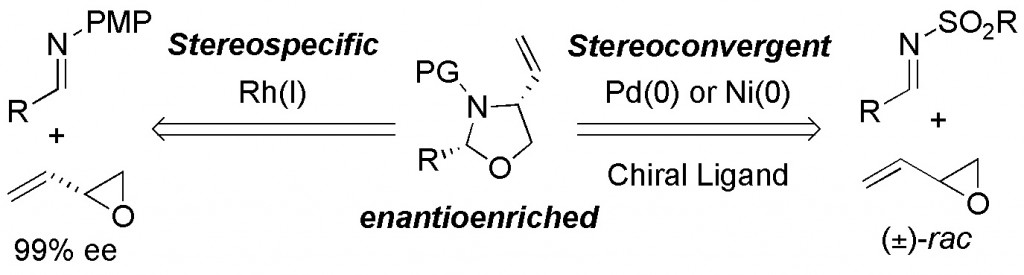
(24) “Oxazolidine Synthesis by Complementary Stereospecific and Stereoconvergent Methods.” M. B. Shaghafi, R. E. Grote, E. R. Jarvo. Org. Lett. 2011, 13, 5188. Link
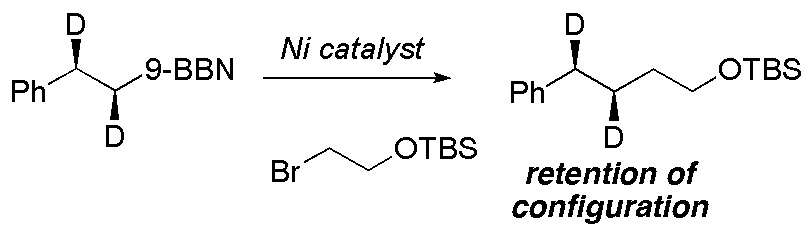
(23) “Stereochemistry of Transmetalation of Alkylboranes in Nickel-Catalyzed Alkyl-Alkyl Cross-Coupling Reactions.” B. L. H. Taylor, E. R. Jarvo. J. Org. Chem. 2011, 76, 7573. Link
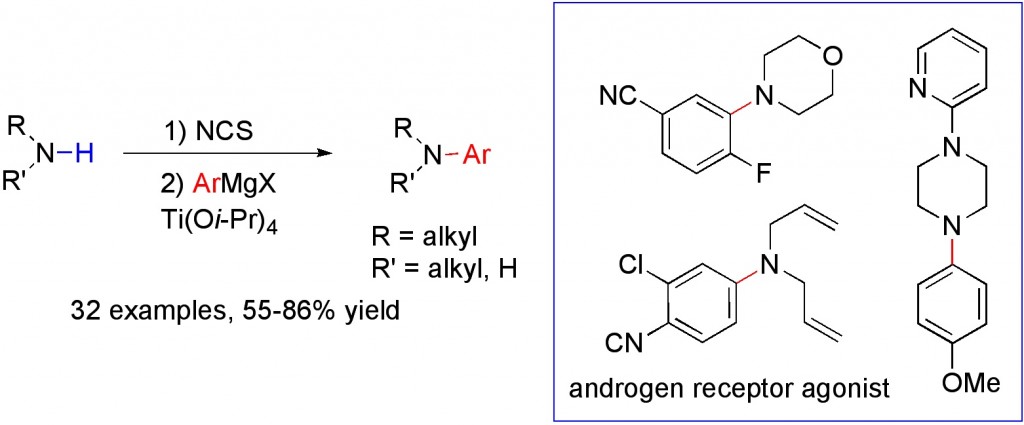
(22) “Titanium-Mediated Amination of Grignard Reagents using Primary and Secondary Amines.” T. J. Barker, E. R. Jarvo. Angew. Chem. Int. Ed. 2011, 50, 8325. Link
Highlighted in Synfacts 2011, 11, 1232.
(21) “Palladium-Catalyzed Cascade Reaction for Synthesis of Substituted Isoindolines.” F. J. Williams, E. R. Jarvo. Angew. Chem. Int. Ed. 2011, 50, 4459. Link
Highlighted in Synfacts 2011, 7, 766.
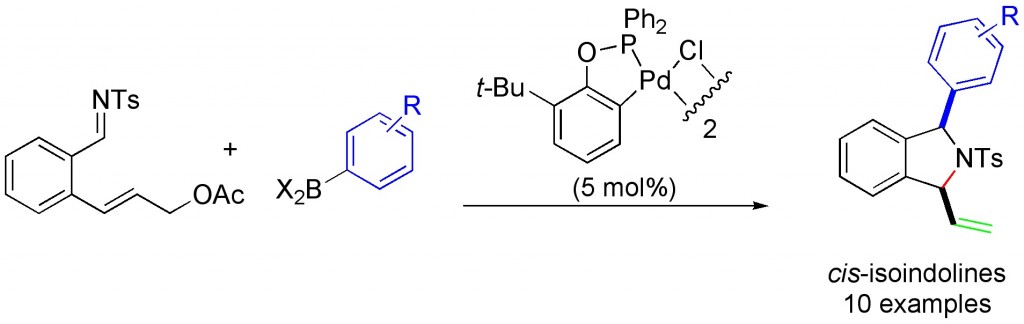
(20) “Enantioselective Silver-Catalyzed Propargylation of Aldimines.” H. M. Wisniewska, E. R. Jarvo. Chem. Sci. 2011, 2, 807-810. Link
Highlighted in Synfacts 2011, 6, 646. Link
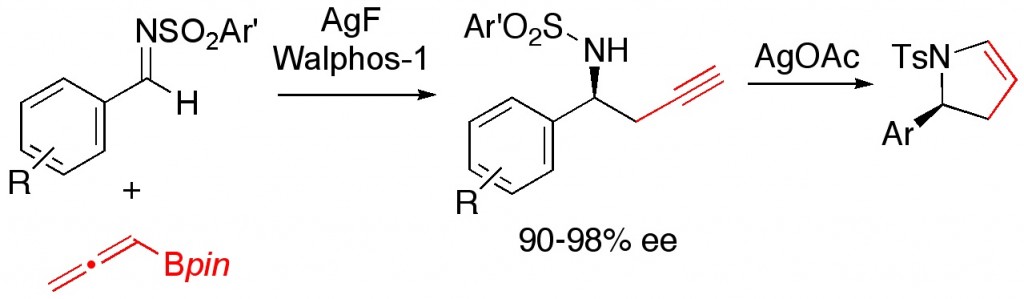
(19) “Stereospecific Nickel-Catalyzed Cross-Coupling Reactions of Alkyl Ethers: Enantioselective Synthesis of Diarylethanes.” B. L. H. Taylor, E. C. Swift, J. D. Waetzig, E. R. Jarvo. J. Am. Chem. Soc. 2011, 133, 389-391. Link
Highlighted in Synfacts 2011, 4, 351.
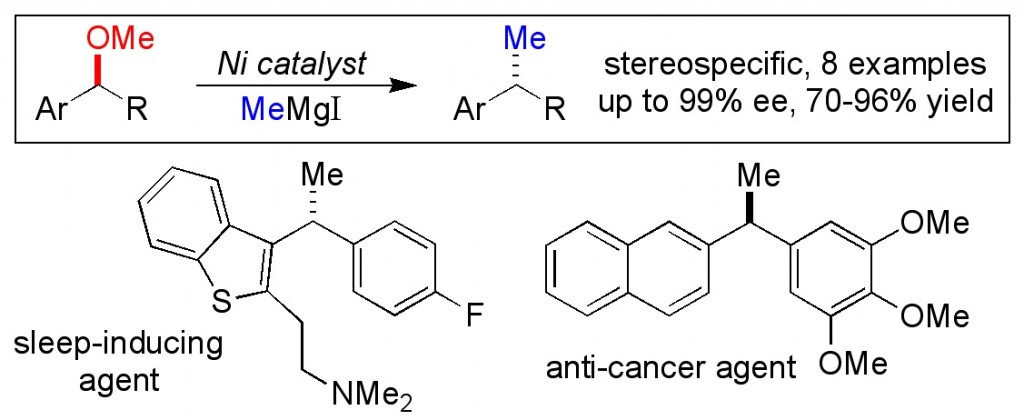
(18) “Diene-Ligated Iridium Complexes as Catalysts for Allylation and Methallylation Reactions of Ketones.” T. J. Barker, E. R. Jarvo. Synthesis 2010, 19, 3259-3262. Link
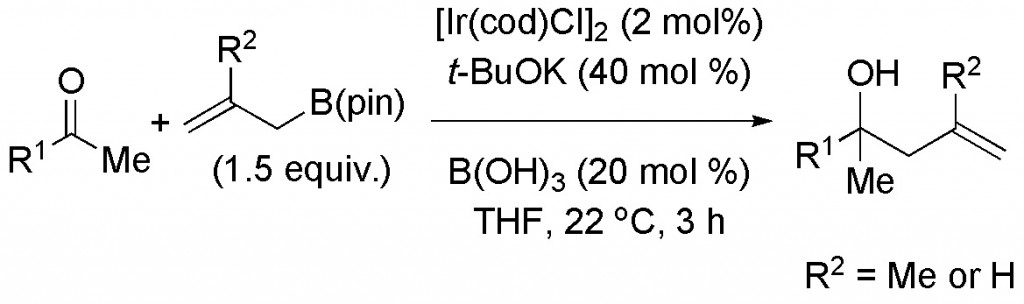
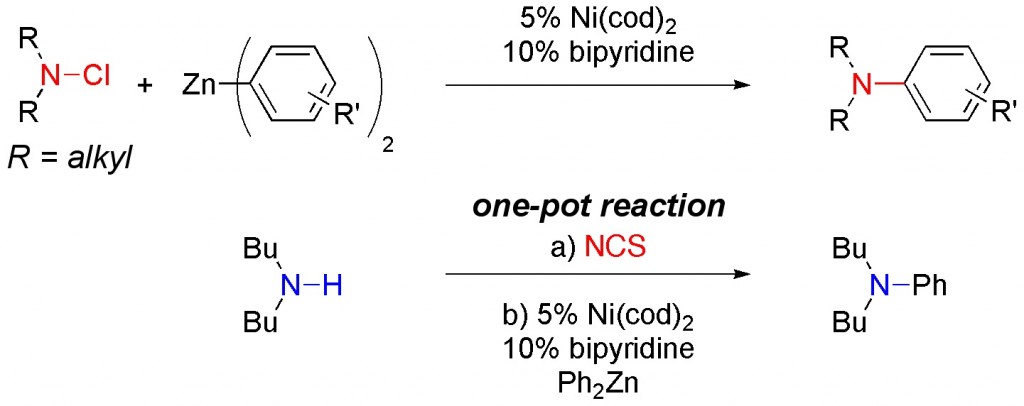
(17) “Umpolung Amination: Nickel-Catalyzed Coupling Reactions of N,N-Dialkyl-N-chloroamines with Diorganozinc Reagents” T. J. Barker, E. R. Jarvo J. Am. Chem. Soc. 2009, 131, 15598-15599. Link
Highlighted in Synfacts 2010, 2, 212.
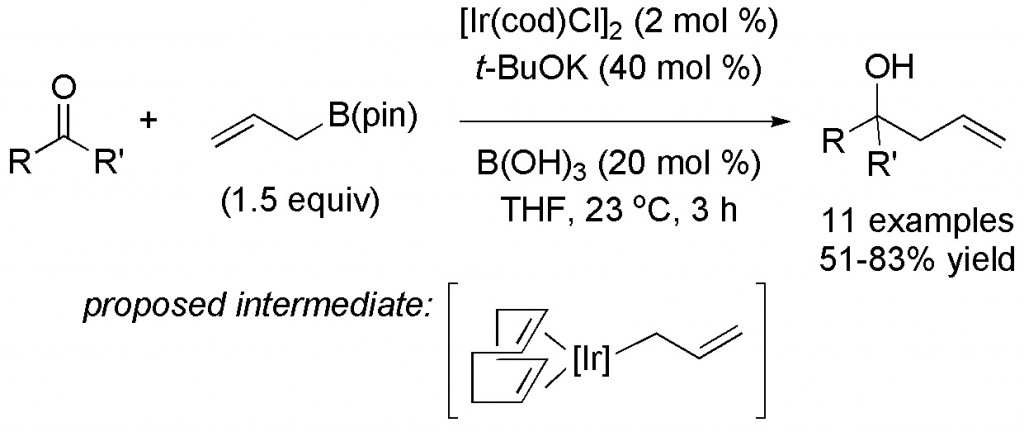
(16) “Diene-Ligated Iridium Catalyst for Allylation Reactions of Ketones and Imines” T. J. Barker, E. R. Jarvo Org. Lett. 2009, 11, 1047-1049. Link
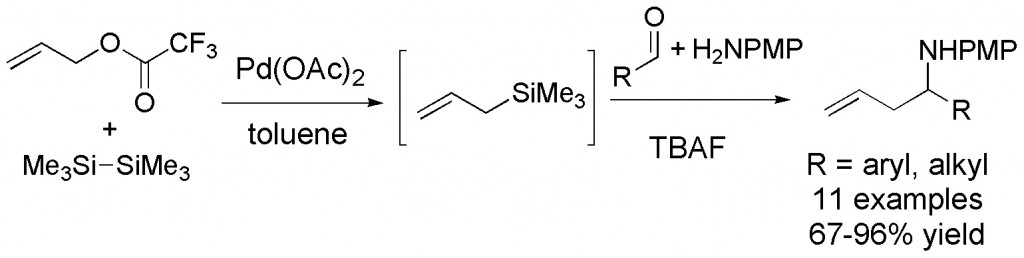
(15) “Palladium-Catalyzed, One-Pot, Three-Component Synthesis of Homoallylic Amines from Aldehyde Anisidine, and Allyl Trifluoroacetate” R. E. Grote, E. R. Jarvo Org. Lett. 2009, 11, 485-488. Link
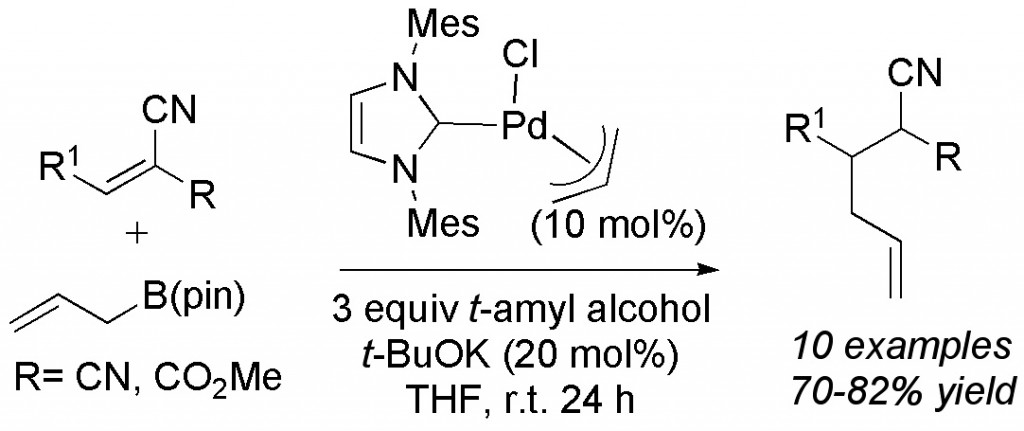
(14) “Conjugate Allylation Reactions of Alkylidene Malononitriles Catalyzed by NHC-ligated Palladium Catalysts.” J.D. Waetzig, E.C. Swift, E.R. Jarvo,Tetrahedron 2009, 65, 3197-3201. Link
(13) “Silver-Catalyzed, Manganese-Mediated Allylation Reactions of Aldehydes and Ketones.” N.T. Barczak, E.R. Jarvo, Eur. J. Org. Chem. 2008, 5507-5510. Link
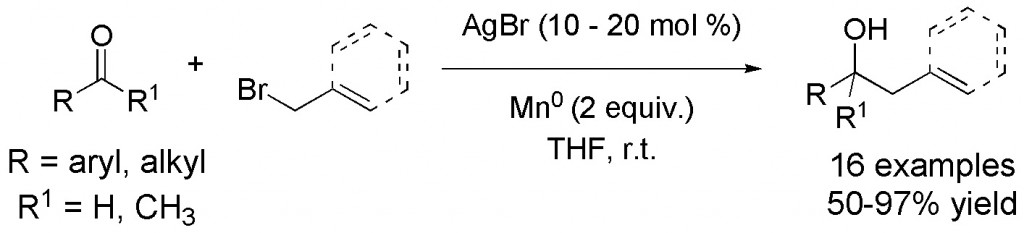
(12) “Palladium-Catalyzed Conjugate Allylation of a,b-Unsaturated N-Acylpyrroles.” M.B. Shaghafi, B.L. Kohn, E.R. Jarvo, Org. Lett. 2008, 10, 4743-4746. Link
Highlighted in Nachrichten aus der Chemie 2009, 57, 6.
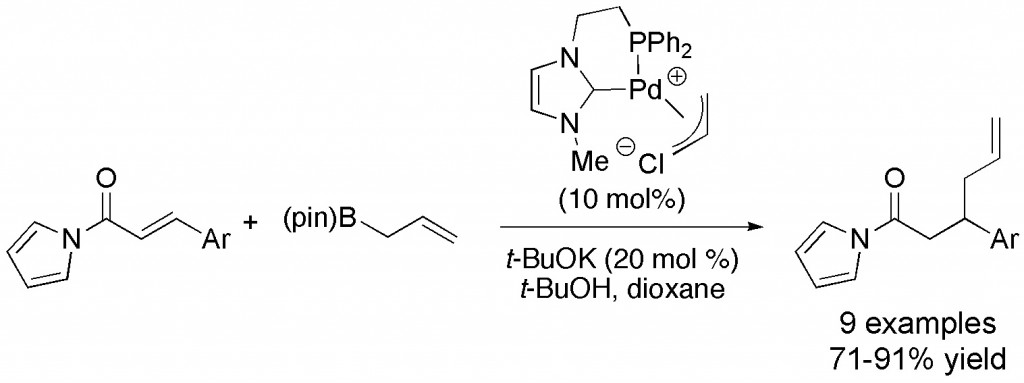
(11) “Catalytic Umpolung Allylation of Aldehydes by π-Allylpalladium Complexes Containing Bidentate N-Heterocyclic Carbene Ligands.” N.T. Barczak, R.E. Grote, E.R. Jarvo, Organometallics 2007, 26, 4863-4865. Link
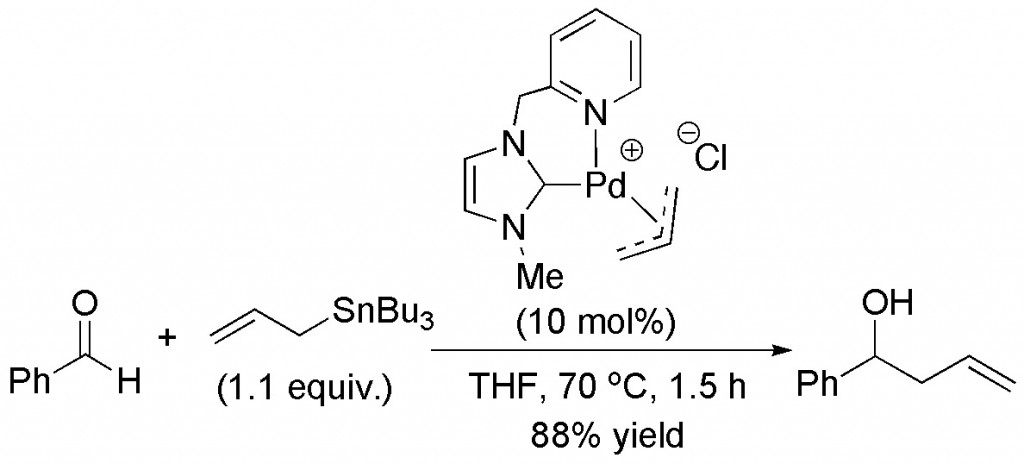
(10) “Efficient Total Syntheses of (-)-Colombiasin A and (-)-Elisapterosin B: Application of the Asymmetric Quinone Diels-Alder Reaction” A.A. Boezio, E.R. Jarvo, B.M. Lawrence, E.N. Jacobsen, Angew. Chem. Int. Ed. 2005, 44, 6046-6050.
(9) “Highly Enantio- and Regioselective Quinone Diels-Alder Reaction Catalyzed by a Tridentate [(Schiff Base CrIII)] Complex.” E.R. Jarvo, B.M. Lawrence, E.N. Jacobsen, Angew. Chem. Int. Ed. 2005, 44, 6043-6046.
(8) “Asymmetric Acylations.” E.R. Jarvo, S.J. Miller, Comprehensive Asymmetric Catalysis, Supplement 1; E.N. Jacobsen, A. Pfaltz, H. Yamamoto, Eds.; Springer-Verlag: Berlin, Heidelberg, 2004; Chapter 43.
(7) “Amino Acids and Peptides as Asymmetric Organocatalysts.” E.R. Jarvo, S.J. Miller, Tetrahedron 2002, 58, 2481-2495.
(6) “Fluorescence-Based Screening of Asymmetric Acylation Catalysts Through Parallel-Enantiomer Analysis. Identification of a Catalysts for Tertiary Alcohol Resolution.” E.R. Jarvo, C.A. Evans, G.T. Copeland, S.J. Miller J. Org. Chem. 2001, 66, 5522-5527.
(5) “Incorporation of Peptide Isosteres into Enantioselective Peptide-Based Catalysts as Mechanistic Probes.” M.M. Vasbinder, E.R. Jarvo, S.J. Miller. Angew. Chem. Int. Ed. 2001, 40, 2824-2827.
(4) “Asymmetric Acylation Reactions Catalyzed by Conformationally Biased Octapeptides.” E.R. Jarvo, M.M. Vasbinder, S.J. Miller. Tetrahedron 2000, 56, 9773-9779.
(3) “A Biomimetic Approach to Asymmetric Acyl Transfer Catalysis.” E.R. Jarvo, G.T. Copeland, N. Papaioannou, P.J. Bonitatebus, Jr., S.J. Miller. J. Am. Chem. Soc. 1999, 121, 11638-11643.
(2) “Minimal Acylase-Like Peptides. Conformational Control of Absolute Stereospecificity.” E.R. Jarvo, G.T. Copeland, S.J. Miller. J. Org. Chem. 1998, 63, 6784-6785.
(1) “High-Pressure Diels-Alder Reactions of Quinone mono-ketals.” E.R. Jarvo, S.R. Boothroyde, M.A. Kerr. Synlett 1996, 879.