Lab technician position, focus on host-microbe interactions
A full or part-time lab technician position is available for a project that is a joint collaboration between the Siryaporn and Gross …
A full or part-time lab technician position is available for a project that is a joint collaboration between the Siryaporn and Gross …
Leora Duong awarded NSF GRFP fellowship for her work relating to Microbial Biology and Biophysics. Congratulations!!!
Just published @ Frontier Med. Tech.: Leora’s Mini-Review on the latest strategies to develop the next generation of antibiotics. https://t.co/hOLdx8vVEs?amp=1
Leora is giving a talk at today’s UCI Immunology Fair around 10:40am “A novel antimicrobial approach: histones and antimicrobial peptides”. Agenda here: http://www.immunology.uci.edu/immunology-fair/agenda.asp
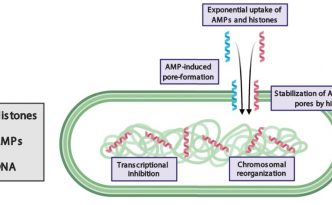
First proposed as antimicrobial agents, histones were later recognized for their role in condensing chromosomes. Histone antimicrobial activity has been reported in innate immune responses. However, how histones kill bacteria has remained elusive. The co-localization of histones with antimicrobial peptides (AMPs) in immune cells suggests that histones may be part of a larger antimicrobial mechanism in vivo. Here we report that histone H2A enters E. coli and S. aureus through membrane pores formed by the AMPs LL-37 and magainin-2. H2A enhances AMP-induced pores, depolarizes the bacterial membrane potential, and impairs membrane recovery. Inside the cytoplasm, H2A reorganizes bacterial chromosomal DNA and inhibits global transcription. Whereas bacteria recover from the pore-forming effects of LL-37, the concomitant effects of H2A and LL-37 are irrecoverable. Their combination constitutes a positive feedback loop that exponentially amplifies their antimicrobial activities, causing antimicrobial synergy. More generally, treatment with H2A and the pore-forming antibiotic polymyxin B completely eradicates bacterial growth.
https://mbio.asm.org/content/11/2/e02730-18.abstract
The rise of antibiotic resistance requires the development of new strategies to combat bacterial infection and pathogenesis. A major direction has been the development of drugs that broadly target virulence. However, few targets have been identified due to the species-specific nature of many virulence regulators. The lack of a virulence regulator that is conserved across species has presented a further challenge to the development of therapeutics. Here, we identify that NADH activity has an important role in the induction of virulence in the pathogen P. aeruginosa. This finding, coupled with the ubiquity of NADH in bacterial pathogens, opens up the possibility of targeting enzymes that process NADH as a potential broad antivirulence approach.
Stay Away, Stay Away – There Is Danger Ahead | by Moselio (Elio) Schaechter | https://t.co/8B9yGdcCji?amp=1
Thank you to those in the lab that helped make the Conference for Undergraduate Women in Physics (CUWiP) an amazing success! http://sites.uci.edu/cuwip2020.
