Metabolic control of m6A RNA modification.

by webmaster
by webmaster
by webmaster

by webmaster
RNA is a crucial genetic material that acts at the central node in gene expression programs. The Lee lab is dedicated to uncovering the involvement of post-transcriptional quality control of RNA molecules, including splicing, chemical modification, transport, stabilization and translation, in rewiring of cellular signaling and metabolism during tumorigenesis. Our ultimate goal is to develop therapeutic approaches and diagnostic tools targeting RNA and metabolic vulnerabilities of cancers.
The kidney is a vital organ responsible for eliminating waste products from the blood while reabsorbing essential nutrients back into the body. Our research focuses on malignant tumor formation in the kidneys, as these tumors not only disrupt kidney function but also have far-reaching consequences on the metabolism of the entire body. Our particular area of interest lies in studying a genetic kidney tumor syndrome known as Tuberous Sclerosis Complex (TSC). This syndrome arises from loss-of-function mutations in the TSC1/2 tumor suppressor genes, leading to hyperactivation of the mechanistic target of rapamycin (mTORC1) signaling, a master regulator of cell growth and proliferation that is overactivated in most human cancers.
Using metabolomic, transcriptomic, and proteomic analyses of genetic and xenograft mouse models, patient-derived cell lines, and patient tissues and serum samples, we elucidate the molecular mechanisms involved in kidney tumor pathogenesis. We found that TSC tumors exhibit overactive lipid metabolism due to the increased expression of fat synthesizing enzymes. Mechanistically, mTORC1 induces the expression of these metabolic enzymes by promoting their RNA splicing and stability. On the other hand, inhibition of these fat synthesis enzymes, combined with extracellular lipid depletion, attenuates TSC tumor growth (Cell 2017; Molecular Cell 2023). Our findings have brought to light the critical role of mTORC1 signaling and RNA processing in the metabolic adaptation of cancer cells, enhancing RNA splicing, stability, and translation of vital metabolic enzymes. Through our research efforts in kidney and other types of human cancers, we hope to contribute to the development of effective treatments and diagnostic tools that can enhance outcomes for cancer patients.
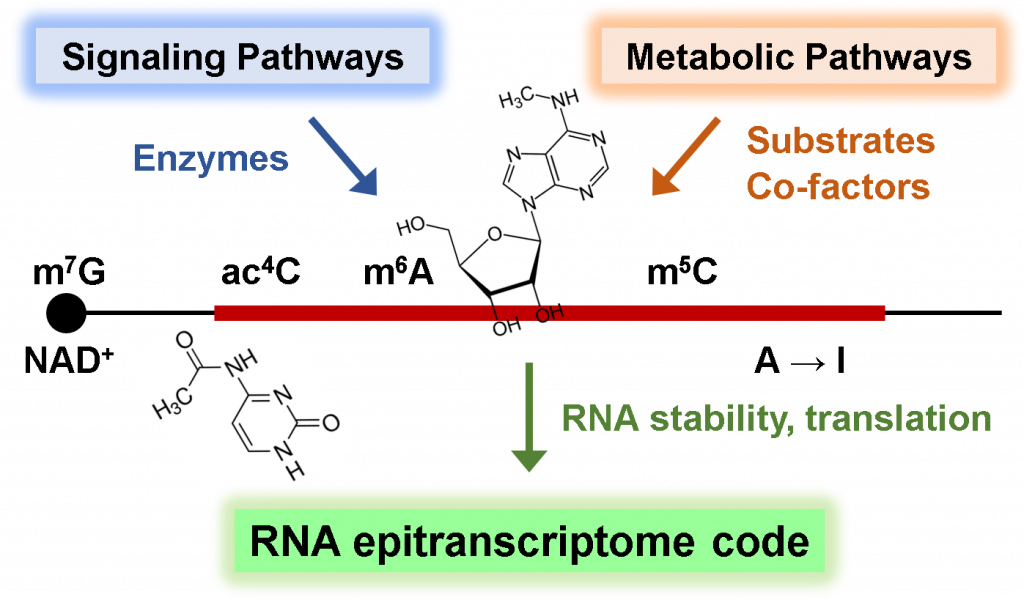
We also explore the fascinating world of RNA epigenetics, where chemical modifications occur on the sugars and bases of RNA molecules. Like DNA and histones undergoing epigenetic modifications, RNA contains an astounding repertoire of over 150 diverse modifications, suggesting the existence of intricate regulatory mechanisms and their crucial functions in gene expression regulation and cell fate decisions. By integrating liquid chromatography-mass spectrometry (LC-MS), RNA modification sequencing, and mathematical modeling, along with in-depth molecular biological and biochemical mechanistic studies, we investigate the signaling and metabolic pathways that control RNA chemical modification, as well as the crosstalk between RNA modification and cellular metabolic processes in normal and malignant cells.
Our research has already yielded exciting results, including our recent discovery of growth factor and oncogenic signaling induced N6-methyl-adenosine (m6A) mRNA modification, and drug resistance mechanisms by m6A-dependent gene expression program (Molecular Cell 2021; JBC 2023). Through our research, we aim to delineate the hidden signaling and metabolic codes embedded within RNA molecules, define how these epigenetic chemical modifications contribute to gene expression programs, and elucidate how their dysregulation leads to human diseases.
(Lee lab trainees; *Equal contribution; #Corresponding author)
Assistant Professor at UC Irvine School of Medicine. Gina got postdoctoral training at Harvard Medical School / Weill Cornell Medicine of Cornell University, and BS and PhD from KAIST / Seoul National University. She is a recipient of Breakout prize, NIH-NCI career transition award, and Mary Kay Ash cancer research grant. She likes walking, jogging, reading books, coffee, and chocolate.
Izabelle graduated from UCSD with a BS in Biochemistry. She is interested in and enjoys recombinant DNA and RNA technology and learning about cancer metabolism. When she’s not in the lab, Izabelle spends her time dancing, crocheting, watching movies, and coffee shop hopping. Although STEM and research are her main pursuits, she often dabbles in the artistic side of herself.
During his PhD at Kangwon National University School of Medicine, Joohwan studied tumor angiogenesis and microRNA biogenesis. He received TSC Alliance postdoctoral fellowship and Vicky Whittemore travel award for his RNA epigenetics research in TSC kidney tumors. While Joohwan’s passion lies in scientific endeavors, he also loves Donkatsu and enjoys meditation.
Ki-Hong focused on studying the comprehensive cell death signaling cascades during his PhD at Chungnam National University. Currently, he delves into the realm of cell survival signaling and metabolism through RNA chemical modification. Aside from his dedication to science, Ki-Hong’s love for basketball becomes evident as he skillfully takes on the roles of shooting guard and small forward.
Yujin completed her PhD at KAIST, where she dedicated her research to unraveling nuclear exosomes and 3D chromatin structures. She has expertise in molecular genetics and bioinformatics and aims to expand her research strength into human cancer and metabolic diseases during her postdoctoral training. Yujin enjoys indoor climbing and spending free time with her dogs. She received a postdoctoral fellowship from the National Research Foundation of Korea.
Temoc is a PhD student co-mentored by Dr. Cholsoon Jang. He is interested in the biochemical elucidation of altered organ and cancer metabolism to develop targeted therapies. When not in lab, Temoc enjoys playing board games and sports with friends and family. He is a recipient of the NIH/NIGMS IMSD and NCI T32 fellowships, and NSF GRFP honorable mention.
Chloe (Eun Jin) is a recent graduate from Vanderbilt University, where she earned her BA in Molecular Cellular Biology. She is planning to attend pharmacy school and is deeply passionate about RNA and its intricate role in cancer biology. In her free time, she enjoys doing pilates, doing Lego, and spending time with her family and friends.
Chloe is a Biological Sciences undergraduate, with a fervid interest in the micro-perspective of cancer mechanisms. She is a recipient of the Rose Hills Foundation and UROP fellowships.
Varvara is an undergraduate student majoring in Biological Sciences. She is interested in nutrient metabolism and public health, and is co-mentored by Dr. Cholsoon Jang; her goal is to understand diet’s influence on health and to translate research discoveries into public health initiatives. In her free time, she enjoys crocheting, climbing, and playing the guitar.
Krystal is an undergraduate student majoring in Biological Sciences. Her research interest is in delineating the pathological characteristics of cancer progression and regression (supported by UROP fellowship). During her free time, she enjoys baking, playing tennis, and spending time with family and friends.
Our lab is located in Sprague Hall, a modern research building dedicated to cancer investigators. We are seeking highly motivated students and postdocs interested in RNA, signaling, and cancer metabolism to join our research team. Please contact the Principal Investigator at ginalee@uci.edu, with a CV, to inquire about potential opportunities.




