Written by Natalie Tran and Edited by Rasheed Majzoub
Antibiotics are an integral part of modern medicine; they dramatically decrease a bacterial population and thereby, reduce the adverse effects of bacterial infections [1]. Because antibiotics are so effective, they were prescribed around 154 million times from 2010 to 2011; however, thirty percent of these prescriptions were unnecessary–they were used to fight viruses instead of bacteria [2]. This overuse and abuse of antibiotics bore a new problem: antibiotic resistance.
Though antibiotics do kill a large majority of a bacterial population, they are incapable of eliminating the whole population: some survivors with antibiotics-circumventing traits remain. As these better-adapted individuals replicate, they pass this trait to their offspring, and the bacterial population along with infection symptoms return [3]. “Despite using a treatment that might have worked ten or twenty years ago when fewer strains were antibiotic-resistant,” comments UCI faculty and phage therapy researcher Dr. Katrine Whiteson, “people are ending up with these nasty infections” [4].
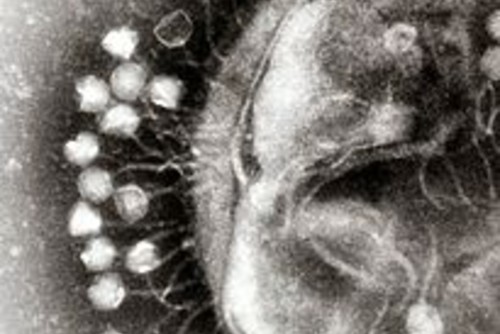
One proposed solution to antibiotic resistance is phage therapy, which deploys phages (viruses) to target specific bacterial populations. Once in the body, the phage seeks out its target bacteria, infects it with the phage’s genome, commands the bacteria to make more phage parts, and eventually bursts the bacteria, killing it [5].
Unlike antibiotics, phage therapy accounts for the bacterial population’s evolution. According to Dr. Katrine Whiteson, bacteria and phages would develop an “arms race.” A phage-infected bacteria “will come up with strategies to counter the phage.” Consequently, “the phage comes up with a new strategy to infect the bacteria” [4]. This “arms race” dynamic is similar to that of countries at war; when one country develops new technology, its enemy will try to match its enemy’s developments.
The bacterial population will have periodic flares of growth as it adapts to the phage’s strategies; hence, one species of phage is not sufficient to permanently contain the bacterial population. Instead, a cocktail of different phage species and even antibiotics would be used to treat a bacterial infection. “If we were to attack the bacteria in a lot of different ways,” says Dr. Whiteson, “then the bacteria cannot mount a resistance” [4]. This approach attacks the bacteria on all fronts, forcing the bacteria to do the near-impossible and adapt to a variety of obstacles simultaneously.
One benefit of phage therapy is the availability and specialization of phages. “For every bacteria in the environment,” says Dr. Whiteson, “there are potentially ten phages ready to infect it. For many strains, it is quite easy [to hunt or collect phages]” [4]. The hardest step is finding a phage with necessary attributes. Phages can be modified for specific purposes through the cloning process. In Dr. Whiteson’s lab, however, phages are modified through the use of “evolution, just selecting phages with the qualities you want, so you don’t have to clone anything” [4].
However, phage therapy is still a developing field, so there are still many unknown factors. One is where phage therapy must be administered to be successful. Although there are many ways to administer phage therapy–for example, through ingestion or through injection into the bloodstream–the scientific community is still unsure on which method is best. On this, Dr. Whiteson comments, “There are people out there who are comparing routes of administration. It is possible that our immune system will respond to the phages differently if you eat them or if they get injected into your bloodstream” [4]. The immune system in the body will have specific responses to phages in the bloodstream and phages in the digestive system; therefore, researchers are investigating the advantages and disadvantages of these administration methods.
Another limitation of phage therapy is clinical trials. Though there have been some promising previous clinical trials, these studies are difficult to access due to language and/or security barriers as with the Polish and Russian trials, or the trials do not comply with specific medical standards, as with French trials [6].
Though there are clear obstacles phage therapy must overcome to be implemented, it appears to be a promising treatment as infection-causing bacterial populations become less responsive to antibiotics. If progress is continued in phage therapy research, it might become a new staple in modern medicine.
References:
- Society, Microbiology. “What Are Antibiotics and How Do They Work?” Microbiology Society, microbiologysociety.org/members-outreach-resources/outreach-resources/antibiotics-unearthed/antibiotics-and-antibiotic-resistance/what-are-antibiotics-and-how-do-they-work.html.
- CDC. “CDC: 1 in 3 Antibiotic Prescriptions Unnecessary.” Centers for Disease Control and Prevention, Centers for Disease Control and Prevention, 1 Jan. 2016, www.cdc.gov/media/releases/2016/p0503-unnecessary-prescriptions.html.
- Center for Disease Control and Prevention. “How Antibiotic Resistance Happens.” Centers for Disease Control and Prevention, Centers for Disease Control and Prevention, 5 Nov. 2019, www.cdc.gov/drugresistance/about/how-resistance-happens.html.
- Whiteson, Katrina. Interview. Conducted by Natalie Tran, 29 January 2020.
- Lin, D.M., Koskella, B., Lin, H.C. (2017). Phage therapy: An alternative to antibiotics in an age of multidrug resistance. World of Gastrointestinal Pharmacology and Therapeutics, 8:162-173.
- Romero-Calle, D., Guimarães Benevides R., Goes-Neto A., Billington C. (2019). Bacteriophages as Alternatives to Antibiotics in Clinical Care. Antibiotics, 8:138.