From: Treseder KK, Lennon JT. 2015. Fungal traits that drive ecosystem dynamics on land. Microbiology and Molecular Biology Reviews 79:243-262.
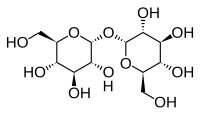
Trehalose is a compatible solute that improves stress tolerance in fungi via several potential mechanisms (Estruch 2000, Francois and Parrou 2001). First, it is thought to substitute for water molecules in cell membranes, protecting them from desiccation and freezing damage (Crowe et al. 1992, Diniz-Mendes et al. 1999, Tibbett et al. 2002, Kandror et al. 2004, Yancey 2005). Second, trehalose may confer thermotolerance (Devirgilio et al. 1994, Deegenaars and Watson 1998, Singer and Lindquist 1998b) by stabilizing proteins during heat shock (Singer and Lindquist 1998a). Third, it may act as a compatible osmolyte (Hare et al. 1998). Accordingly, a number of studies have documented increases in trehalose concentrations in fungi in response to environmental stress (Deegenaars and Watson 1998, Estruch 2000, Francois and Parrou 2001, Tibbett et al. 2002). Trehalose concentrations can vary among fungi (Tibbett et al. 2002), and have been primarily studied in yeast (e.g., Singer and Lindquist 1998b, Francois and Parrou 2001).
Trehalose can represent a significant trade-off for fungi, because it requires C that could otherwise be allocated to growth or metabolism (Schimel et al. 2007). It is a high-energy compound (Estruch 2000, Francois and Parrou 2001). Moreover, it can represent as much as 20% of fungal biomass (Singer and Lindquist 1998b). Indeed, Schimel et al. (2007) estimated that the C cost of producing stress-resistance compounds such as trehalose during a single drought event can reach as much as 6% of an ecosystem’s annual net primary productivity.
Functional genes related to trehalose production in fungi
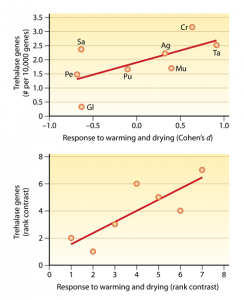
In a high-latitude boreal forest, Allison et al. (2010) used greenhouses to simultaneously increase soil temperature and decrease soil moisture, and then assessed changes in fungal community composition. In this ecosystem, ambient soil conditions are quite cold and dry, so the manipulations exacerbated drought while ameliorating temperature extremes (Allison et al. 2010). For the current study, we re-analyzed their community data and found that phyla/subphyla that responded most positively to warming and drying were those that carried higher frequencies of trehalase genes. This response is consistent with our understanding of the role of trehalose in resistance to desiccation in fungi (Schimel et al. 2007).
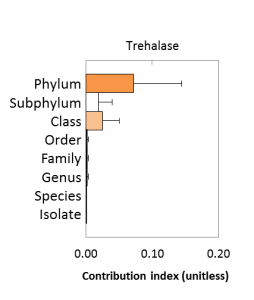
The genetic capacity for trehalose production varies most at the phylum level:
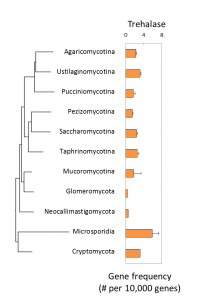
Phylogenetic distribution of trehalose genes:
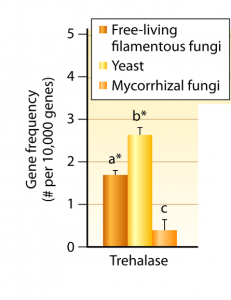
Trehalose genes are most common in yeast:
Allison, S. D., K. L. McGuire, and K. K. Treseder. 2010. Resistance of microbial and soil properties to warming treatment seven years after boreal fire. Soil Biol Biochem 42:1872-1878.
Crowe, J. H., F. A. Hoekstra, and L. M. Crowe. 1992. Anhydrobiosis. Annual Review of Physiology 54:579-599.
Deegenaars, M. L. and K. Watson. 1998. Heat shock response in psychrophilic and psychrotrophic yeast from Antarctica. Extremophiles 2:41-49.
Devirgilio, C., T. Hottiger, J. Dominguez, T. Boller, and A. Wiemken. 1994. The role of trehalose synthesis for the aquisition of thermotolerance in yeast. 1. Genetic evidence that trehalose is a thermoprotectant. Eur J Biochem 219:179-186.
Diniz-Mendes, L., E. Bernardes, P. S. de Araujo, A. D. Panek, and V. M. F. Paschoalin. 1999. Preservation of frozen yeast cells by trehalose. Biotechnology and Bioengineering 65:572-578.
Estruch, F. 2000. Stress-controlled transcription factors, stress-induced genes and stress tolerance in budding yeast. FEMS Microbiol Rev 24:469-486.
Francois, J. and J. L. Parrou. 2001. Reserve carbohydrates metabolism in the yeast Saccharomyces cerevisiae. FEMS Microbiol Rev 25:125-145.
Grigoriev, I. V., R. Nikitin, S. Haridas, A. Kuo, R. Ohm, R. Otillar, R. Riley, A. Salamov, X. Zhao, F. Korzeniewski, T. Smirnova, H. Nordberg, I. Dubchak, and I. Shabalov. 2014. MycoCosm portal: gearing up for 1000 fungal genomes. Nucleic Acids Research 42:D699-704.
Hare, P. D., W. A. Cress, and J. Van Staden. 1998. Dissecting the roles of osmolyte accumulation during stress. Plant Cell and Environment 21:535-553.
Kandror, O., N. Bretschneider, E. Kreydin, D. Cavalieri, and A. L. Goldberg. 2004. Yeast adapt to near-freezing temperatures by STRE/Msn2,4-dependent induction of trehalose synthesis and certain molecular chaperones. Molecular Cell 13:771-781.
McGuire, K. L., E. Bent, J. Borneman, A. Majumder, S. D. Allison, and K. K. Treseder. 2010. Functional diversity in resource use by fungi. Ecology 91:2324-2332.
Schimel, J., T. C. Balser, and M. Wallenstein. 2007. Microbial stress-response physiology and its implications for ecosystem function. Ecology 88:1386-1394.
Singer, M. A. and S. Lindquist. 1998a. Multiple effects of trehalose on protein folding in vitro and in vivo. Molecular Cell 1:639-648.
Singer, M. A. and S. Lindquist. 1998b. Thermotolerance in Saccharomyces cerevisiae: the Yin and Yang of trehalose. Trends Biotechnol 16:460-468.
Tibbett, M., F. E. Sanders, and J. W. G. Cairney. 2002. Low-temperature-induced changes in trehalose, mannitol and arabitol associated with enhanced tolerance to freezing in ectomycorrhizal basidiomycetes (Hebeloma spp.). Mycorrhiza 12:249-255.
Yancey, P. H. 2005. Organic osmolytes as compatible, metabolic and counteracting cytoprotectants in high osmolarity and other stresses. Journal of Experimental Biology 208:2819-2830.