Competing Enantioselective Conversion (CEC) Method
Assigning the absolute configuration of a molecule is an essential part of the chemical characterization process. There are several methods currently available which include the advanced Mosher method, X-ray crystallography, and circular dichroism. These methods often require significant amounts of material, take an extensive period of time, or require high level equipment to perform. Our group has demonstrated that kinetic resolution reagents can be used to assign the absolute configuration of certain functional groups.
This Competing Enantioselective Conversion (CEC) method works by reacting each enantiomer of a kinetic resolution reagent with an enantiopure substrate and measuring conversion. Once the fast reaction is determined, the difference in conversion results in configuration assignment based on an empirically derived mnemonic. The CEC method bares many advantages over the commonly used methods to assign absolute configuration. Each CEC method is exceptionally simple to perform, does not require exotic equipment, and uses minuscule amounts of material. The reactions are performed on milligram to micrograms of material and the results can simply be analyzed by NMR, mass spectrometry, or even TLC.
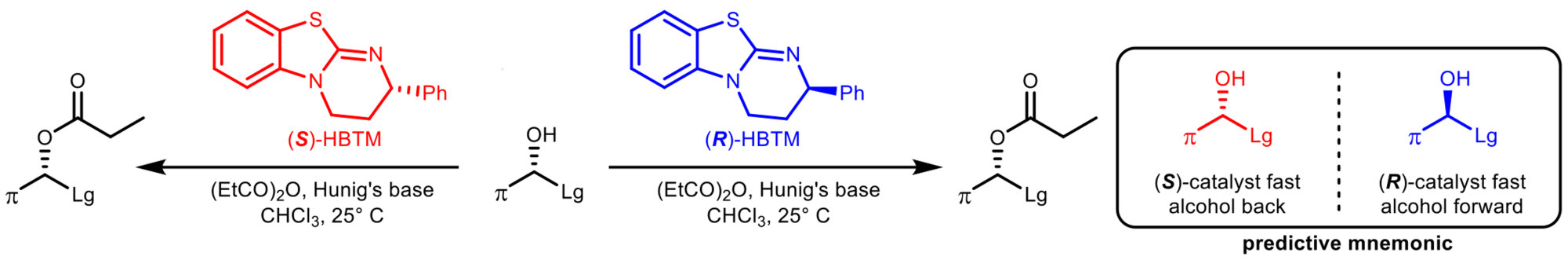
Assigning the Absolute Configuration of Alcohols, Oxazolidinones, and Lactams Using HBTM
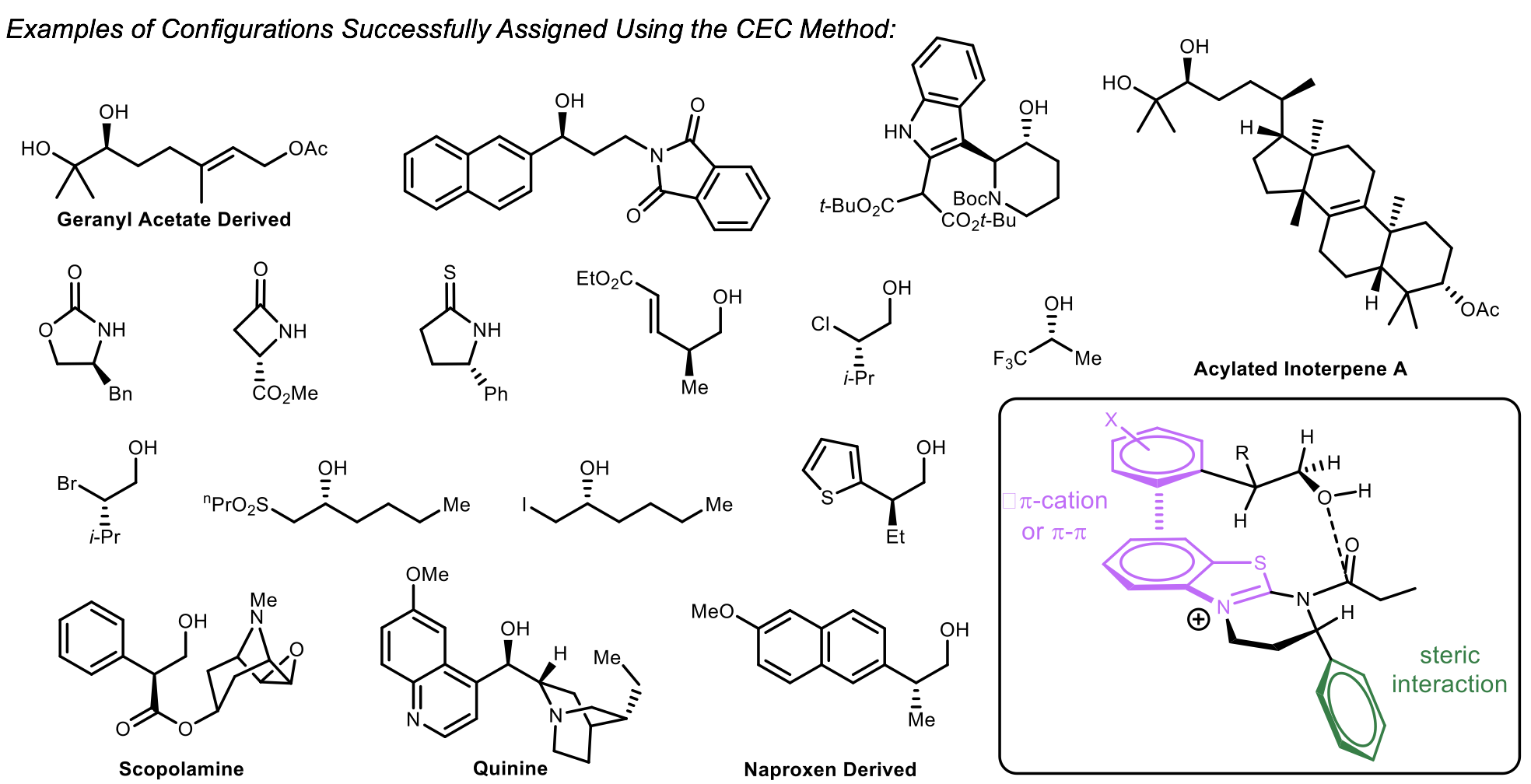
Our laboratory has been able to successfully assign the configuration of many alcohols, oxazolidinones, and lactams using Birman’s HBTM catalysts. The method requires running two parallel acylation reactions using (R)-HBTM as an acyl transfer catalyst in one reaction and (S)-HBTM in the other. Each reaction is monitored by NMR or TLC to determine the faster reaction. Using the predictive mnemonic, the absolute configuration is assigned. Many successful substrates were assigned including Scopolamine, Quinine, and acylated inoterpene A.

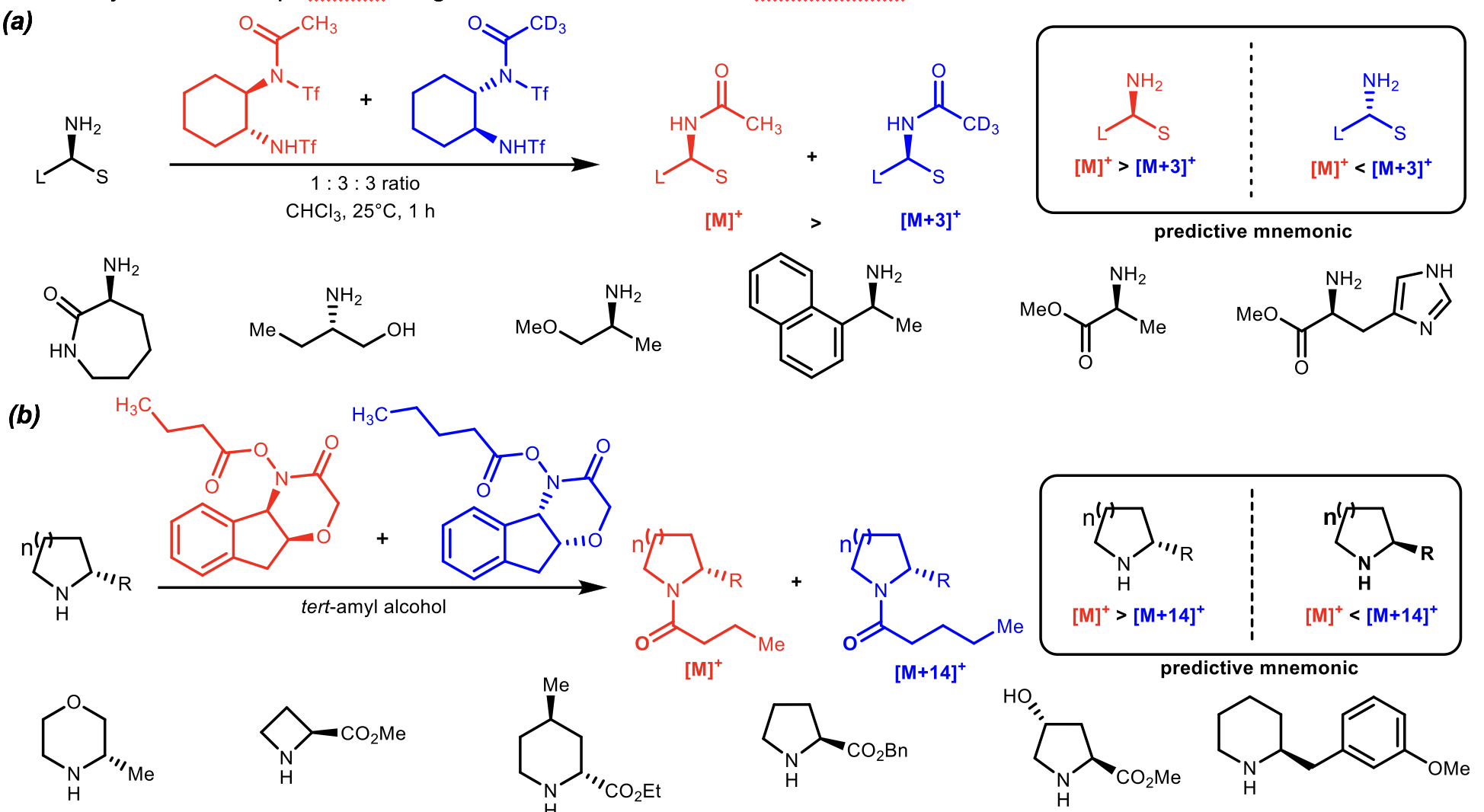
Assigning the Absolute Configuration of Primary and Secondary Amines
The absolute stereochemistry of primary and secondary amines were successfully assigned using a similar strategy to that of the alcohols. In the case of primary amines, pseudoenantiomers of Mioskowski’s reagents were added in a one to one molar ratio to a single reaction with the desired amine. The conversion was analyzed by electrospray ionization mass spectrometry and the configuration was assigned based on an empirically derived predictive mnemonic. The same strategy was utilized for secondary amines except Bode’s reagents were used instead of Mioskowski’s.
A New Approach Towards the Synthesis of Substituted Pyridines
Pyridines are common heterocyclic motifs found in natural products, as well as biologically relevant synthetic targets (e.g Nexium, Reyataz, Pradaxa). Due to their popularity/abundance within the synthetic community, many methods exist to generate substituted pyridine compounds. These methods can be split into two categories. The first is the functionalization of halogenated pyridine starting materials through cross coupling reactions. These methods usually require the use of expensive starting materials. The second is the synthesis of pyridines through cyclization reactions. These methods are usually limited to a specific substitution pattern and can require complex starting materials or costly reagents. Our method aims to provide a reliable synthesis of a diverse scope of substituted pyridines through a concise procedure.