Natural Product Synthesis
Natural products have inspired the development of new synthetic methodologies, while providing important templates for the discovery of novel drug candidates. The total synthesis of natural products has historically demonstrated the ability of synthetic chemists to not only make complex molecules, but also problem solve and adapt, similar to the way that Nature does. Our lab continues to contribute in the area of total synthesis by demonstrating novel approaches to a variety of natural products. Along the way, we have also developed new methods, corrected and assigned chemical structures, and generated unnatural analogues to better understand the biological activity of these molecules.
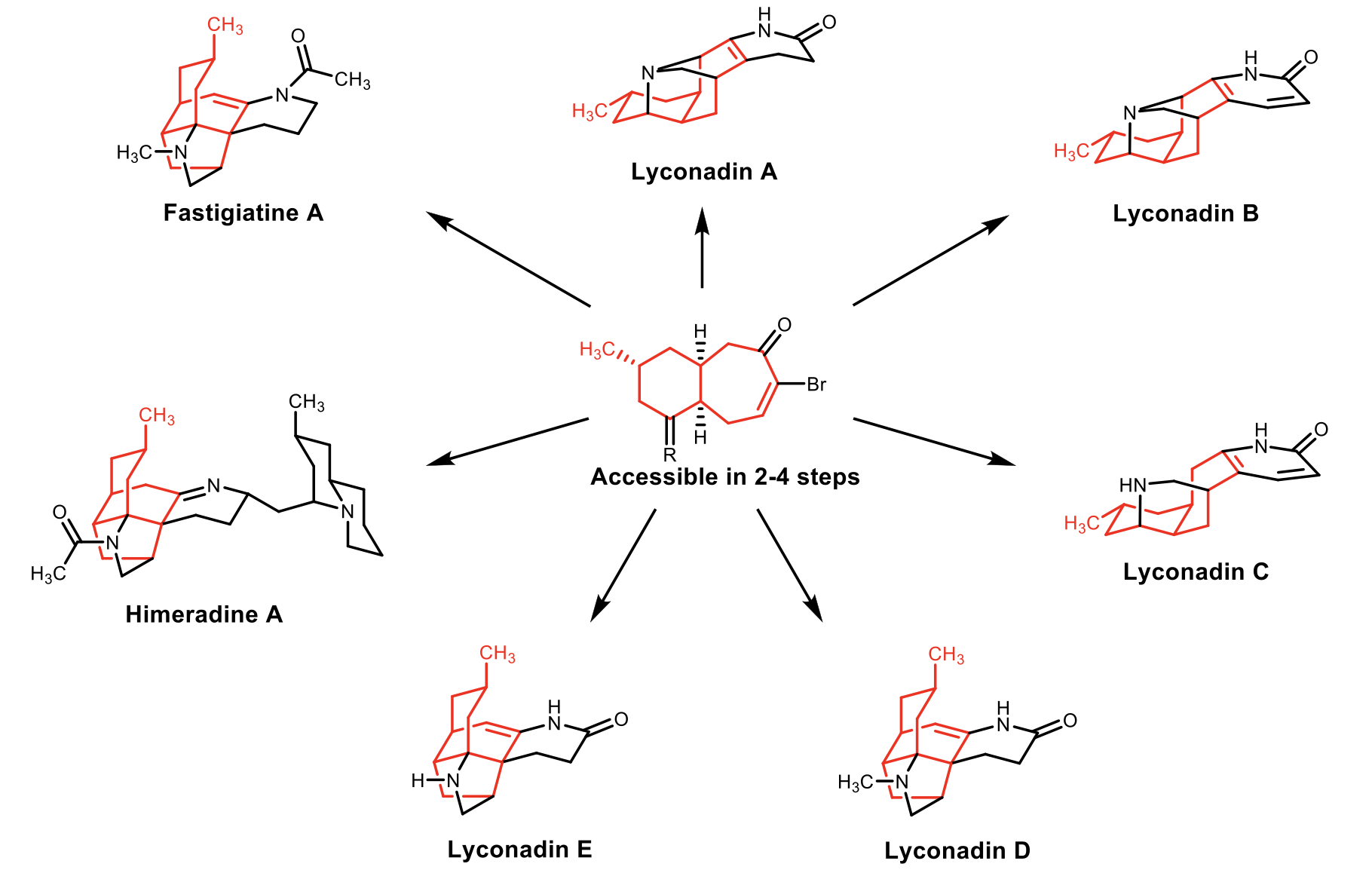
A Unified Approach Towards the Lycopodium Alkaloids
The Lycopodium alkaloids are a diverse group of structurally complex natural products that have been shown to possess interesting biological activities, ranging from neurotropic to anticancer properties. The Rychnovsky lab recently published a concise total synthesis of (+)-fastigiatine. We now look to utilize a common 6,7-bicyclic bromoenone in the synthesis of six other members of the Lycopodium alkaloid family, himeridine A and lyconadin A-E.
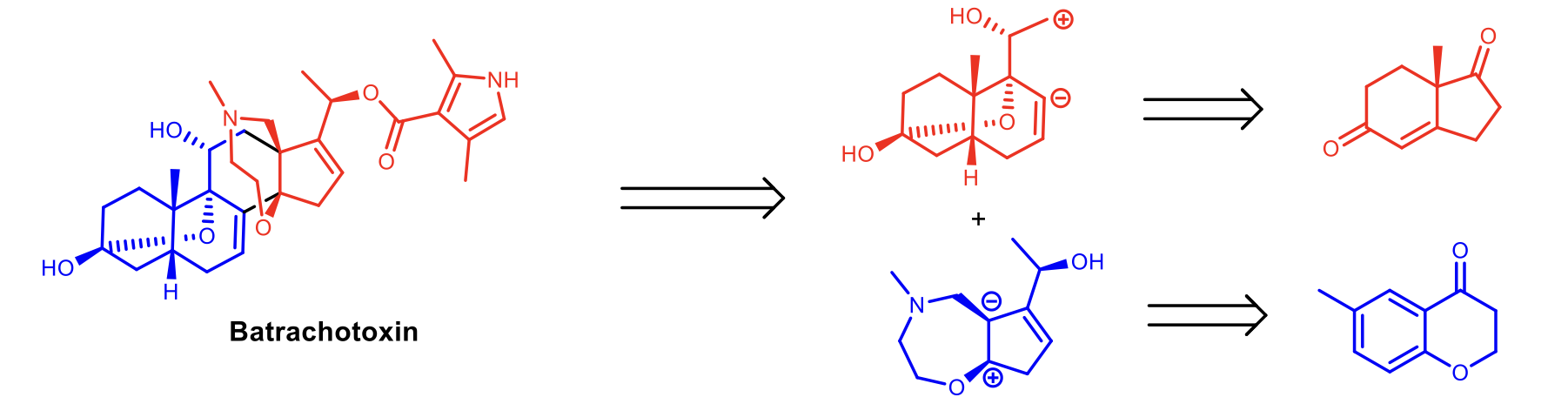
Total Synthesis of Batrachotoxin
Our group has had a long-standing interest in synthesizing the complex steroidal alkaloid batrachotoxin. With an LD50 of 0.2-2 µg/kg, it is the most potent non-peptidyl neurotoxin known. As a highly selective agonist of voltage-gated sodium ion channels, analogues of this steroid may be promising candidates for treating diabetes, epilepsy, and congenital pain-related conditions. Our current synthetic approach is focused on a C-ring disconnection, which simplifies the molecule to an elaborated cis-fused AB decalin, and a 5,7-oxazepane DE ring system that is unique to the batrachotoxin family of natural products.
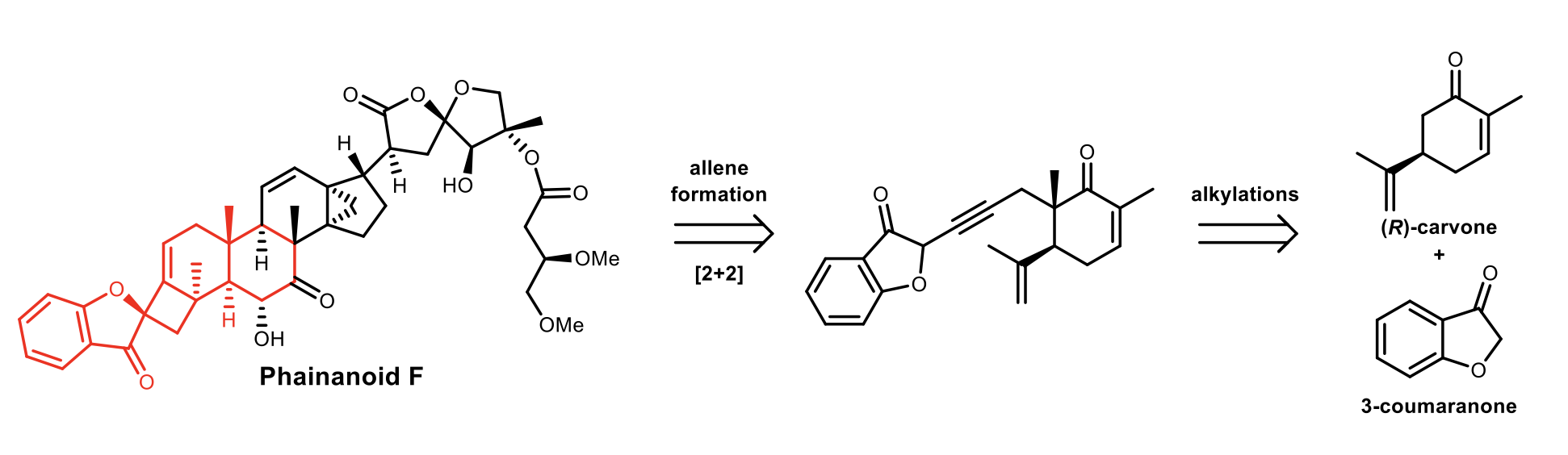
Synthesis of the Western Portion of Phainanoid
Phainanoid F (PNF) was first reported in 2014, which featured a complex 13,30-cyclodammarane triterpenoid core, with an unprecedented 3H-spiro[benzofuran-2,1′-cyclobutan]-3-one motif. This compound was reported to be a highly potent immunosuppressant, with an IC50 of 2.04 nM and 1.6 nM for ConA-induced T-cell and LPS-induced B-cell proliferation, respectively. We envisioned the benzofuran spirocycle to be installed through key [2+2] cyclization of an allene and isopropenyl group. This intermediate would come from two alkylations of (R)-carvone followed by a coupling with 3-coumaranone.