The optical microscopy resources at the Beckman Laser Institute and Medical Clinic, designated for basic research and pre-clinical applications, are used by our NLOM Lab and Principal Investigators at UCI to investigate and understand the biology of various health conditions and diseases. Most of these collaboration and service projects are cutting edge and have needs that go beyond simple turnkey experiments. Dr. Tatiana Krasieva works closely with the users providing her expertise for the study design, data analysis and interpretation. Our resource calendar can be accessed here.
Most recent projects
Brain Endothelial Erythrophagocytosis and Hemoglobin Transmigration Across Brain Endothelium: Implications for Pathogenesis of Cerebral Microbleeds
Investigators: Mark J. Fisher, MD, and Rachita K. Sumbria, PhD, Departments of Neurology and Pathology & Laboratory Medicine, UCI
It has long been believed that a tear or rupture of a brain blood vessel is the cause of cerebral microbleeds. This study found that brain endothelial cells, the cells that line blood vessels of the brain, have the capacity for engulfing red blood cells and depositing them outside the blood vessels and into the substance of the brain, without requiring a disruption of the vasculature. This study identifies thus a new direction related to the cause and consequences of cerebral microbleeds.

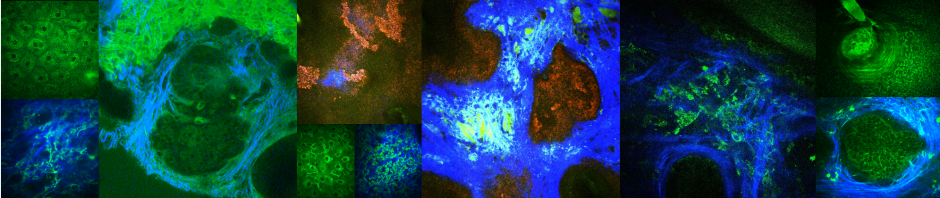
Ex-vivo images of mouse brains showing GFP-positive brain endothelium (green) and PKH-26-labeled RBC (red). (A, Top): PBS-RBC remain in the blood vessels (white arrows heads). (A, Bottom): t-BHP-treated RBC are inside (white arrow head) and outside the blood vessels (yellow arrowhead), and also partially extravasated into the brain (blue arrow head). Orthogonal view showing PBS-treated RBC within the blood vessel (B, Top) and t-BHP-treated RBC partially and completely outside the blood vessel (B, Bottom). Scale bar = 50μm.
Related publications/news:
Chang et al, Frontiers in Neuroscience 12:279 (2018)
Nevogenesis and carcinogenesis in BRAF mutant mouse model
Investigator: Anand Ganesan, MD, PhD, Department of Dermatology, UC Irvine
Melanoma, a tumor resistant to therapy in late stages, is curable by excision when caught early. Early melanomas can be difficult to distinguish from benign, pigmented “moles”, i.e. melanocytic nevi; this leads to unnecessary excision of many normal nevi while early melanomas are often missed. Clinical and experimental data show that ~90% of nevi are initiated when melanocytes acquire an activating mutation in the BRAF oncogene, the same oncogenic mutation observed in >60% of melanomas. Mouse models of Braf activation have recently been developed that mimic human nevus formation, and also produce melanomas either at low frequency or when additional oncogenic mutations are added. Studying nevi and melanomas in such models can potentially shed light on how the same initiating event can lead to a lesion that reversibly arrests itself and regresses, and one that grows out of control.
We combine a conditional genetic mouse model with a non-invasive imaging technology, multiphoton microscopy (MPM), which enables us to observe the dynamics of transformed melanocytes in live animals at first (P21) and second (P50) synchronized telogen (this is a point in the hair cycle where hairs can be easily removed and won’t immediately re-grow).
Nevogenesis and carcinogenesis in mouse model in-vivo. P50 (days after birth)/ t178/ATR WT BrafV600E treated with PBS (control) and p50/ t176/ATR het BrafV600E treated with clodronate since p30 to deplete macrophages. True focus (projection of 0 to 100 µm volume of skin, reconstructed from the stack of images taken at the 3 µm z-step, FOV 636µmx636µm starting from the surface). Olympus 20x water immersion objective, n.a.=1.0; Green -GFP expressing melanocytes, red -tdTomato– keratinocytes, cyan – SHG of collagen; Excitation wavelengths: 488nm and 920 nm, emission bp 390-465 nm, bp 500-530 nm and bp 565-651 nm). All three channels were acquired simultaneously.
Related publications: